| |
| Names | |
|---|---|
| IUPAC name 1,1,2,2-tetrachloro-1,2-difluoroethane | |
| Other names 1,1,2,2-Tetrachloro-1,2-difluoroethane; 1,2-Difluorotetrachloroethane; Freon 112; 1,2-Difluoro-1,1,2,2-tetrachloroethane; ; sym-Tetrachlorodifluoroethane; R 112; CFC-112; 1,1,2,2-Tetrachlorodifluoroethane; | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.851 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1078 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2Cl4F2 |
| Molar mass | 203.82 g·mol |
| Appearance | clear liquid or white solid |
| Density | 1.634 g/mL |
| Melting point | 23.8 °C (74.8 °F; 296.9 K) |
| Boiling point | 92.8 °C (199.0 °F; 365.9 K) |
| Solubility in water | 0.012% |
| Refractive index (nD) | 1.4130 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H320 |
| Precautionary statements | P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, P362+P364 |
| Related compounds | |
| Related compounds | CFC-112a |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
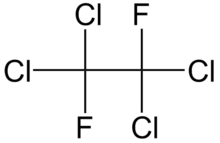
Tetrachloro-1,2-difluoroethane is a chlorofluorocarbon known as Freon 112, CFC-112 or R-112. It has a symmetrical structure CCl2FCCl2F and so can be called symmetrical tetrachlorodifluoroethane. "Symmetrical" may also be abbreviated to "s-" or "sym-". In contrast an asymmetrical isomer has formula CCl3CClF2.
Production
CFC-112 can be made in a reaction with hydrogen fluoride with hexachloroethane or tetrachloroethane with extra chlorine. This reaction occurs with an aluminium fluoride catalyst with some extra iron, nickel and chromium at 400°C. With the extra metal in the catalyst yield of the isomer can be 98% compared with the unsymmetrical isomer.
Mixed with perfluorooctane, it is a solvent for polydimethylsiloxane.
CFC-112 can be prepared as a mixture with other hydrochlorofluorocarbons from trichloroethylene and anhydrous hydrogen fluoride when electric current is passed through.
When CFC-11 is packaged with alcohol in a metal container, a free radical reaction can result in production of CFC-112.
Properties
Critical properties are critical temperature 278°C, critical pressure 4.83 MPa at a density of 0.754 g/cc.
Tetrachloro-1,2-difluoroethane is not flammable.
Tetrachloro-1,2-difluoroethane, like other chlorofluorocarbon compounds reacts violently with sodium, potassium or barium.
Tetrachloro-1,2-difluoroethane is not very toxic, and the lethal dose is estimated at 25 g/kg. It is not carcinogenic.
Use
Tetrachlorodifluoroethane (mixture of isomers) has been used as a veterinary medicine to treat parasites such as liver fluke in sheep (Fasciola hepatica).
MIL-C-8638 is a military specification for a cleaning solvent that contained tetrachlorodifluoroethane, trichlorotrifluoroethane, and isopropyl alcohol. It was used to clean aircraft oxygen systems.
Tetrachlorodifluoroethane can be used as an intermediate in the manufacture of tetrachloroethylene.
Atmosphere
In the atmosphere of Earth, anthropogenic tetrachloro-1,2-difluoroethane has been found to occur. Between 2017 and 2020 levels, were about 0.52 parts per trillion (ppt). Levels rose from the 1970s till about 1995.
References
- ^ Bruno, Thomas J. (1990). Spectroscopic Library for Alternative Refrigerant Analysis. U.S. Department of Commerce, National Institute of Standards and Technology. p. 5.
- Vecchio, M; Groppelli, G; Tatlow, J.C. (July 1974). "Studies on a vapour-phase process for the manufacture of chlorofluoroethanes". Journal of Fluorine Chemistry. 4 (2): 117–139. doi:10.1016/S0022-1139(00)82507-5.
- Crescenzi, V.; Flory, P. J. (January 1964). "Configuration of the Poly-(dimethylsiloxane) Chain. II. Unperturbed Dimensions and Specific Solvent Effects". Journal of the American Chemical Society. 86 (2): 141–146. doi:10.1021/ja01056a006.
- Polisena, C.; Liu, C. C.; Savinell, R. F. (1 December 1982). "Experimental Study of Electrochemical Fluorination of Trichloroethylene". Journal of the Electrochemical Society. 129 (12): 2720–2724. Bibcode:1982JElS..129.2720P. doi:10.1149/1.2123655.
- ^ Fully halogenated chlorofluorocarbons. Geneva: World Health Organization. 1990. p. 15. hdl:10665/39345. ISBN 9241571136.
- McKellar, Quintin A.; Kinabo, Ludovick D. B. (1 July 1991). "The pharmacology of flukicidal drugs". British Veterinary Journal. 147 (4): 306–321. doi:10.1016/0007-1935(91)90003-6. PMID 1913127.
- Poliakoff, M. Z (1973). "Solvent Cleaners - Where and how to Use Them". Cleaning Stainless Steel. ASTM International. p. 37.
- Gottlieb, Robert (22 April 2013). Reducing Toxics: A New Approach To Policy And Industrial Decisionmaking. Island Press. p. 243. ISBN 978-1-61091-102-3.
- Dunse, Bronwyn L.; Derek, Nada; Fraser, Paul J.; Krummel, Paul B. (June 2021). "Australian and Global Emissions of Ozone Depleting Substances" (PDF). CSIRO.