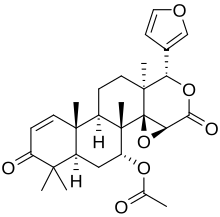
| |
| Names | |
|---|---|
| IUPAC name Gedunin | |
| Systematic IUPAC name nonadec-13-en-19-yl] acetate | |
Other names
| |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references |
Gedunin is a pentacyclic triterpenoid with the molecular formula C28H34 O7. It is most notably found in Azadirachta indica, but is a constituent of several other plants. Gedunin shows therapeutic potential in the treatment of leukemia, and Parkinson's disease.
Natural occurrence
Azadirachta indica is the most notable source of gedunin, but it has also been found in the following plants:
- Cedrela fissilis
- Cedrela odorata
- Cedrela salvadorensis
- Entandrophragma angolense
- Khaya grandifoliola
- Melia azedarach
- Toona sinensis
- Xylocarpus granatum
References
- Hallur, Gurulingappa; Sivramakrishnan, Apoorba; Bhat, Sujata V. (2002-08-01). "Three New Tetranortriterpenoids from Neem Seed Oil". Journal of Natural Products. 65 (8): 1177–1179. doi:10.1021/np0105174. ISSN 0163-3864. PMID 12193026.
- Kikuchi, Takashi; Ishii, Koichi; Noto, Taisuke; Takahashi, Akitomo; Tabata, Keiichi; Suzuki, Takashi; Akihisa, Toshihiro (2011-04-25). "Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem)". Journal of Natural Products. 74 (4): 866–870. doi:10.1021/np100783k. ISSN 1520-6025. PMID 21381696.
- Rane, Anand; Rajagopalan, Subramanian; Ahuja, Manuj; Thomas, Bobby; Chinta, Shankar J.; Andersen, Julie K. (March 2018). "Hsp90 Co-chaperone p23 contributes to dopaminergic mitochondrial stress via stabilization of PHD2: Implications for Parkinson's disease". Neurotoxicology. 65: 166–173. doi:10.1016/j.neuro.2018.02.012. ISSN 1872-9711. PMC 5857252. PMID 29471019.
- Zhou, Heying; Li, Fengxia; Li, Yanli (November 2022). "Anti-Cancer Activity of Gedunin by Induction of Apoptosis in Human Gastric Cancer AGS Cells". Applied Biochemistry and Biotechnology. 194 (11): 5322–5332. doi:10.1007/s12010-022-04001-8. ISSN 1559-0291. PMID 35759172. S2CID 250065297.
- Chianese, Giuseppina; Yerbanga, Serge R.; Lucantoni, Leonardo; Habluetzel, Annette; Basilico, Nicoletta; Taramelli, Donatella; Fattorusso, Ernesto; Taglialatela-Scafati, Orazio (2010-08-27). "Antiplasmodial Triterpenoids from the Fruits of Neem, Azadirachta indica". Journal of Natural Products. 73 (8): 1448–1452. doi:10.1021/np100325q. ISSN 0163-3864. PMID 20669933.
- Leite, Ana; Ambrozin, Alessandra; Fernandes, João; Vieira, Paulo; da Silva, Maria; de Albuquerque, Sérgio (December 2008). "Trypanocidal Activity of Limonoids and Triterpenes from Cedrela fissilis". Planta Medica. 74 (15): 1795–1799. doi:10.1055/s-0028-1088323. ISSN 0032-0943. PMID 18991203. S2CID 260248307.
- Campos, Angela M.; Oliveira, Francisco S.; Machado, Maria Iracema L.; Matos, Francisco J.A.; Braz-Filho, Raimundo (January 1991). "Triterpenes from Cedrela odorata". Phytochemistry. 30 (4): 1225–1229. doi:10.1016/S0031-9422(00)95206-3.
- Carvalho, Paulo S.; Napolitano, Hamilton B.; Camargo, Ademir J.; Silva, Valter H.C.; Ellena, Javier A.; Rocha, Waldireny C.; Vieira, Paulo C. (January 2012). "X-ray diffraction and theoretical investigation of the Gedunin crystal structure". Journal of Molecular Structure. 1008: 83–87. doi:10.1016/j.molstruc.2011.11.028.
- Céspedes, Carlos L.; Calderón, José S.; Lina, Laura; Aranda, Eduardo (2000-05-01). "Growth Inhibitory Effects on Fall Armyworm Spodoptera frugiperda of Some Limonoids Isolated from Cedrela spp. (Meliaceae)". Journal of Agricultural and Food Chemistry. 48 (5): 1903–1908. doi:10.1021/jf990443q. ISSN 0021-8561. PMID 10820113.
- Okorie, Domingo A.; Taylor, David A.H. (January 1977). "Triterpenes from the seed of Entandrophragma species". Phytochemistry. 16 (12): 2029–2030. doi:10.1016/0031-9422(77)80123-4.
- Bickii, Jean; Njifutie, Njikam; Ayafor Foyere, Johnson; Basco, Leonardo K; Ringwald, Pascal (January 2000). "In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae)". Journal of Ethnopharmacology. 69 (1): 27–33. doi:10.1016/S0378-8741(99)00117-8. PMID 10661881.
- Khalid, Sami A.; Farouk, Asim; Geary, Timothy G.; Jensen, James B. (February 1986). "Potential antimalarial candidates from African plants: An in vitro approach using Plasmodium falciparum". Journal of Ethnopharmacology. 15 (2): 201–209. doi:10.1016/0378-8741(86)90156-X. PMID 3520157.
- Mitsui, Kumiko; Saito, Hiroaki; Yamamura, Ryota; Fukaya, Haruhiko; Hitotsuyanagi, Yukio; Takeya, Koichi (2007). "Apotirucallane and Tirucallane Triterpenoids from Cedrela sinensis". Chemical and Pharmaceutical Bulletin. 55 (10): 1442–1447. doi:10.1248/cpb.55.1442. ISSN 0009-2363. PMID 17917286.
- Mitsui, Kumiko; Saito, Hiroaki; Yamamura, Ryota; Fukaya, Haruhiko; Hitotsuyanagi, Yukio; Takeya, Koichi (2006-09-01). "Hydroxylated Gedunin Derivatives from Cedrela sinensis". Journal of Natural Products. 69 (9): 1310–1314. doi:10.1021/np068021f. ISSN 0163-3864. PMID 16989525.
- Uddin, Shaikh J.; Nahar, Lutfun; Shilpi, Jamil A.; Shoeb, Mohammad; Borkowski, Tomasz; Gibbons, Simon; Middleton, Moira; Byres, Maureen; Sarker, Satyajit D. (August 2007). "Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line In Vitro". Phytotherapy Research. 21 (8): 757–761. doi:10.1002/ptr.2159. PMID 17450509. S2CID 22441840.
- Li, Min-Yi; Yang, Xiao-Bo; Pan, Jian-Yu; Feng, Gang; Xiao, Qiang; Sinkkonen, Jari; Satyanandamurty, Tirumani; Wu, Jun (2009-12-28). "Granatumins A−G, Limonoids from the Seeds of a Krishna Mangrove, Xylocarpus granatum". Journal of Natural Products. 72 (12): 2110–2114. doi:10.1021/np900625w. ISSN 0163-3864. PMID 19888743.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |