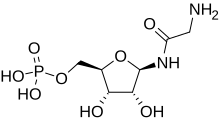
| |
| Names | |
|---|---|
| IUPAC name (1R)-1,4-Anhydro-1-glycinamido-D-ribitol 5-(dihydrogen phosphate) | |
| Systematic IUPAC name methyl dihydrogen phosphate | |
| Other names
Glycineamide ribotide, GAR | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H15N2O8P |
| Molar mass | 286.177 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Glycineamide ribonucleotide (or GAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from GAR.
GAR is the product of the enzyme phosphoribosylamine—glycine ligase acting on phosphoribosylamine (PRA) to combine it with glycine in a process driven by ATP. The reaction, EC 6.3.4.13 forms an amide bond:
- PRA + glycine + ATP → GAR + ADP + Pi
The biosynthesis pathway next adds a formyl group from 10-formyltetrahydrofolate to GAR, catalysed by phosphoribosylglycinamide formyltransferase in reaction EC 2.1.2.2 and producing formylglycinamide ribotide (FGAR):
- GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate
See also
References
- R. Caspi (2009-01-13). "Pathway: 5-aminoimidazole ribonucleotide biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-02.
- Zhang, Y.; Morar, M.; Ealick, S.E. (2008). "Structural biology of the purine biosynthetic pathway". Cellular and Molecular Life Sciences. 65: 3699–3724. doi:10.1007/s00018-008-8295-8. PMC 2596281. PMID 18712276.
- Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6. S2CID 234897784.
- Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition. 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMC 3147014. PMID 20886485.
- R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-10.
- Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society. 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMC 4753784. PMID 26237670.
- ^ Welin, Martin; Grossmann, Jörg Günter; Flodin, Susanne; Nyman, Tomas; Stenmark, Pål; Trésaugues, Lionel; Kotenyova, Tetyana; Johansson, Ida; Nordlund, Pär; Lehtiö, Lari (2010). "Structural studies of tri-functional human GART". Nucleic Acids Research. 38 (20): 7308–7319. doi:10.1093/nar/gkq595. PMC 2978367. PMID 20631005.
| Nucleotide metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| purine metabolism |
| ||||||||||
| pyrimidine metabolism |
| ||||||||||
