| |
| Names | |
|---|---|
| IUPAC name Methyl glycinate hydrochloride | |
| Systematic IUPAC name Methyl 2-aminoacetate hydrochloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.024.672 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
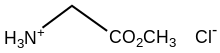
| Chemical formula | C3H8ClNO2 |
| Molar mass | 125.55 g·mol |
| Appearance | white solid |
| Melting point | 175–176 °C (347–349 °F; 448–449 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Glycine methyl ester hydrochloride is the organic compound with the formula Cl. A white, water-soluble solid, it is the hydrochloride of the methyl ester of the amino acid glycine.
Synthesis and reactions
Glycine methyl ester hydrochloride can be prepared by treatment of glycine with 2 equivalents of trimethylsilyl chloride, followed by the addition of methanol.
Upon treatment with base, the salt converts to glycine methyl ester.
Glycine methyl ester (and other esters of glycine) are not shelf-stable, tending to polymerize when stored at room temperature or convert to diketopiperazine. The hydrochloride is shelf-stable.
References
- "Glycine methyl ester hydrochloride". pubchem.ncbi.nlm.nih.gov. Retrieved 24 January 2022.
- Li, Jiabo; Sha, Yaowu (2008). "A Convenient Synthesis of Amino Acid Methyl Esters". Molecules. 13 (5): 1111–1119. doi:10.3390/molecules13051111. PMC 6245331. PMID 18560331.
- White, James D.; Kranemann, Christian L.; Kuntiyong, Punlop (2002). "4-Methoxycarbonyl-2-methyl-1,3-oxazole". Org. Synth. 79: 244. doi:10.15227/orgsyn.079.0244.
- ^ Myers, Andrew G.; Gleason, James L. (1999). "Asymmetric Synthesis of α-Amino Acids by the Alkylation of Pseudoephedrine Glycinamide: L-Allylglycine and N-BOC-l-Allylglycine". Organic Syntheses. 76: 57. doi:10.15227/orgsyn.076.0057.