| |
| Names | |
|---|---|
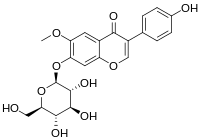
| IUPAC name 7-(β-D-Glucopyranosyloxy)-4′-hydroxy-6-methoxyisoflavone | |
| Systematic IUPAC name 3-(4-Hydroxyphenyl)-6-methoxy-7-{oxy}-4H-1-benzopyran-4-one | |
| Other names Glycitein 7-O-glucoside | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H22O10 |
| Molar mass | 446.408 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Glycitin (glycitein 7-O-glucoside) is an isoflavone found in soy, and remains to various degrees in soy products like tofu, soymilk and soy sauce. Although glycitin has its own health associated properties (below), it can be transformed to glycitein by human intestinal flora by the action of beta-glucosidases.
Properties
Some interesting effects of glycitin include human dermal fibroblast cell proliferation and migration via TGF‐β signaling, glycitin treatment produces anti-photoaging effects such as collagen type I and collagen type III increase at both the mRNA and protein levels. Other noted effects decreased elastase, and decreased β‐galactosidase activation. In conjunction with 4′,6,7-trimethoxyisoflavone (TMF), an isoflavone that promotes fibroblast migration but not proliferation, wound healing and anti-scarring activity (reorganization and wound fibrosis inhibition) were significantly and synergistically boosted in both in vivo mice and in vitro.
References
- Hsiao, Yu-Hsuan; Yu, Chia-Jung; Li, Wen-Tai; Hsieh, Jung-Feng (2015). "Coagulation of β-conglycinin, glycinin and isoflavones induced by calcium chloride in soymilk". Scientific Reports. 5: 13018. Bibcode:2015NatSR...513018H. doi:10.1038/srep13018. PMC 4542527. PMID 26260443.
- "Health Benefits of Naturally Brewed Soy Sauce". 2018-04-04.
- "Human Metabolome Database: Showing metabocard for Glycitin (HMDB0002219)".
- Kim, Young Mee; Huh, Jung Sik; Lim, Yoongho; Cho, Moonjae (2015). "Soy Isoflavone Glycitin (4'-Hydroxy-6-Methoxyisoflavone-7-D-Glucoside) Promotes Human Dermal Fibroblast Cell Proliferation and Migration via TGF-β Signaling". Phytotherapy Research. 29 (5): 757–769. doi:10.1002/ptr.5313. PMID 25758427. S2CID 206430410.
- Kim, Joungmok; Yang, Goowon; Kim, Yeji; Kim, Jin; Ha, Joohun (2016). "AMPK activators: Mechanisms of action and physiological activities". Experimental & Molecular Medicine. 48 (4): e224. doi:10.1038/emm.2016.16. PMC 4855276. PMID 27034026.
| Isoflavones and their glycosides | |
|---|---|
| Isoflavones | |
| O-methylated isoflavones | |
| Glycosides | |
| Prenylated isoflavones | |
| Pyranoisoflavones | |
| Derivatives | |
| Synthetic | |
| Soy (Glycine max) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General |  | ||||||||||||
| Soy-based dishes | |||||||||||||
| Plant milk | |||||||||||||
| Meat analogues | |||||||||||||
| Sauces and condiments |
| ||||||||||||
| Other foods | |||||||||||||
| Biochemicals |
| ||||||||||||
| Companies | |||||||||||||
| Other |
| ||||||||||||