
| |
| Names | |
|---|---|
| IUPAC name (1aR,4R,4aR,7bS)-1,1,4,7-Tetramethyl-1a,2,3,4,4a,5,6,7b-octahydrocyclopropaazulene | |
| Other names (-)-α-Gurjunene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.996 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H24 |
| Molar mass | 204.357 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Gurjunene, also known as (-)-α-gurjunene, is a natural carbotricyclic sesquiterpene that is most commonly found in gurjun balsam, an essential oil compound extracted from plants of the genus Dipterocarpus. The following reaction that synthesizes gurjunene can be catalyzed by alpha-gurjunene synthase:
(2E,6E)-farnesyl diphosphate (–)-α-gurjunene + diphosphate
Related compounds
Several related compounds are known, including β-gurjunene and γ-gurjunene.
 β-Gurjunene
β-Gurjunene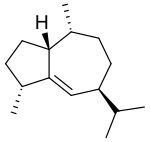 γ-Gurjunene
γ-Gurjunene
References
- "GURJUN BALSAM (GURJUNENE) MD". www.ventos.com. Retrieved 2023-07-20.
- PubChem. "alpha-Gurjunene". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-07-20.
- Schmidt CO, Bouwmeester HJ, Bülow N, König WA (April 1999). "Isolation, characterization, and mechanistic studies of (-)-α-gurjunene synthase from Solidago canadensis". Archives of Biochemistry and Biophysics. 364 (2): 167–77. doi:10.1006/abbi.1999.1122. PMID 10190971.
 (–)-α-gurjunene +
(–)-α-gurjunene +