| |
| Names | |
|---|---|
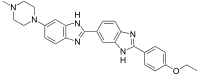
| Preferred IUPAC name 2′-(4-Ethoxyphenyl)-6-(4-methylpiperazin-1-yl)-1H,3′H-2,5′-bi-1,3-benzimidazole | |
| Other names Hoechst 33342; Hoe 33342 | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.041.523 |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H28N6O |
| Molar mass | 452.562 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Bisbenzimide (Hoechst 33342) is an organic compound used as a fluorescent stain for DNA in molecular biology applications. Several related chemical compounds are used for similar purposes and are collectively called Hoechst stains.
Application
Bisbenzimide tends to bind to adenine–thymine-rich regions of DNA and can decrease its density. Bisbenzimide mixed with DNA samples can then be used to separate DNA according to their AT percentage using a cesium chloride (CsCl) gradient centrifugation.

References
External links
- Fluorescence Spectra: http://www.fluorophores.tugraz.at/substance/463