| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
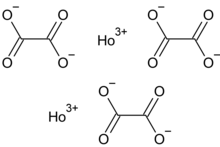
| Chemical formula | Ho2(C2O4)3 |
| Appearance | yellow crystals (heptahydrate) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Holmium(III) oxalate is the oxalate salt of holmium, with the chemical formula Ho2(C2O4)3. It exists in anhydrous and hydrated forms.
Properties
Holmium(III) oxalate decahydrate decomposes in heat to obtain the dihydrate, which is further heated to obtain the anhydrous form, and finally holmium(III) oxide is obtained. It reacts with hydrochloric acid to obtain H·6H2O.
References
- Wendlandt, W. W. (1959). "Thermal Decomposition of Rare Earth Metal Oxalates". Analytical Chemistry. 31 (3): 408–410. doi:10.1021/ac60147a024. ISSN 0003-2700.
- Moebius, R.; Matthes, F. (1964). "The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium". Zeitschrift für Chemie. 4 (6): 234–235.
| Holmium compounds | |
|---|---|
| Compounds of the oxalate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||