| |
| Names | |
|---|---|
| IUPAC name (2S,2′S)-4,4'-Disulfanediylbis(2-aminobutanoic acid) | |
| Other names L-Homocystine; L-4,4′-Dithiobis(2-aminobutanoic acid) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ECHA InfoCard | 100.009.966 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
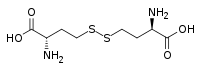
| Chemical formula | C8H16N2O4S2 |
| Molar mass | 268.35 g·mol |
| Appearance | colorless solid |
| Melting point | 281–284 °C (538–543 °F; 554–557 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Homocystine is the organosulfur compound with the formula (HO2CCH(NH2)CH2CH2S)2. It is disulfide derived from oxidation of homocysteine. Its relationship with homocysteine is analogous to the relationship between cystine and cysteine.
References
- "L-Homocystine". Sigma-Aldrich.
- Jackson, Peter; Stanley, Keith; Luzio, J. Paul (1986). "Specific fluorescent detection of disulphide-bridged peptides on thin-layer chromatograms". Biochemical Society Transactions. 14 (4): 750–751. doi:10.1042/bst0140750.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |