| Hydrohalite | |
|---|---|
| General | |
| Category | Halide mineral |
| Formula (repeating unit) | NaCl·2H2O |
| IMA symbol | Hhl |
| Strunz classification | 3.BA.05 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | P21/c |
| Identification | |
| Colour | Colourless or white |
| Diaphaneity | Transparent |
Hydrohalite is a mineral that occurs in saturated halite brines at cold temperatures (below 0.1 °C). It was first described in 1847 in Dürrnberg, Austria. It exists in cold weather.
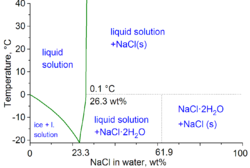
Hydrohalite has a high nucleation energy, and solutions will normally need to be supercooled for crystals to form. The cryohydric point is at −21.2 °C (−6.2 °F). Above this temperature, liquid water saturated with salt can exist in equilibrium with hydrohalite. Hydrohalite has a strong positive temperature coefficient of solubility, unlike halite. Hydrohalite decomposes at 0.1°C, giving a salty brine and solid halite. Under pressure, hydrohalite is stable between 7,900 and 11,600 atmospheres pressure. The decomposition point increases at the rate of 0.007K per atmosphere (for 1–1000 atmospheres). The maximum decomposition temperature is at 25.8°C under 9400 atmospheres. Above this pressure the decomposition point goes down.
Ceres
Hydrohalite was discovered on Ceres by Dawn, suggesting an early ocean, possibly surviving as a relict ocean.
References
- Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Braitsch, O. (1971). "The Stability Conditions of Salt Minerals". Salt Deposits Their Origin and Composition. Springer. pp. 42–44. doi:10.1007/978-3-642-65083-3_2. ISBN 978-3-642-65085-7.
- De Sanctis, M.C., Ammannito, E., Raponi, A. et al. Fresh emplacement of hydrated sodium chloride on Ceres from ascending salty fluids. Nat Astron 4, 786–793 (2020). https://doi.org/10.1038/s41550-020-1138-8
This article about a specific halide mineral is a stub. You can help Misplaced Pages by expanding it. |