| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methylbutane | |
| Other names Isopentane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1730723 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.039 |
| EC Number |
|
| Gmelin Reference | 49318 |
| MeSH | isopentane |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1265 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H12 |
| Molar mass | 72.151 g·mol |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 616 mg mL |
| Melting point | −161 to −159 °C; −258 to −254 °F; 112 to 114 K |
| Boiling point | 27.8 to 28.2 °C; 81.9 to 82.7 °F; 300.9 to 301.3 K |
| Vapor pressure | 76.992 kPa (at 20 °C) |
| Henry's law constant (kH) |
7.2 nmol Pa kg |
| UV-vis (λmax) | 192 nm |
| Refractive index (nD) | 1.354 |
| Viscosity | 0.214 cP (at 20 °C) |
| Thermochemistry | |
| Heat capacity (C) | 164.85 J K mol |
| Std molar entropy (S298) |
260.41 J K mol |
| Std enthalpy of formation (ΔfH298) |
−179.1–−177.3 kJ mol |
| Std enthalpy of combustion (ΔcH298) |
~ 3.3 MJ mol, 19,664 Btu/lb |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H224, H301, H302, H305, H336, H411 |
| Precautionary statements | P210, P261, P273, P301+P310, P331 |
| NFPA 704 (fire diamond) |
 |
| Flash point | −51 °C (−60 °F; 222 K) |
| Autoignition temperature |
420 °C (788 °F; 693 K) |
| Explosive limits | 1.4–8.3% |
| Related compounds | |
| Related alkanes | |
| Related compounds | 2-Ethyl-1-butanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
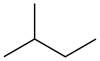
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane, but it is a significant component of natural gasoline.
Nomenclature
The traditional name isopentane was still retained in the 1993 IUPAC recommendations, but is no longer recommended according to the 2013 recommendations. The preferred IUPAC name is the systematic name 2-methylbutane. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Uses
Isopentane is used in a closed loop in geothermal power production to drive turbines.
Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology.
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common petroleum-derived gasoline that is condensed from natural gas. It has a substantially higher octane rating (RON 93.7) than n-pentane (61.7), and therefore there is interest in conversion from the latter.
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names 'isobutane', 'isopentane' and 'neopentane' are no longer recommended.
- James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 doi:10.1021/ie990588m
- Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_073.pub2
- ^ Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States, Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.
- Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- Panico, R. & Powell, W. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
- Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. HS Orka HF Energy Plant IV Archived 2014-10-18 at the Wayback Machine
- "Animal Resources Program - the Office of the Vice President for Research | UAB".
- Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". International Journal of Thermophysics, volume 35, pages 1450–1464. doi:10.1007/s10765-014-1718-x
External links
- International Chemical Safety Card 1153
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
| Alkanes | |
|---|---|
| |