| |||
| Names | |||
|---|---|---|---|
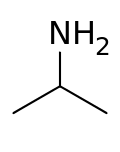
| Preferred IUPAC name Propan-2-amine | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 605259 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.783 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | 2-propylamine | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1221 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C3H9N | ||
| Molar mass | 59.112 g·mol | ||
| Appearance | Colourless liquid | ||
| Odor | "Fishy"; ammoniacal | ||
| Density | 688 mg mL | ||
| Melting point | −95.20 °C; −139.36 °F; 177.95 K | ||
| Boiling point | 31 to 35 °C; 88 to 95 °F; 304 to 308 K | ||
| Solubility in water | Miscible | ||
| log P | 0.391 | ||
| Vapor pressure | 63.41 kPa (at 20 °C) | ||
| Refractive index (nD) | 1.3742 | ||
| Thermochemistry | |||
| Heat capacity (C) | 163.85 J K mol | ||
| Std molar entropy (S298) |
218.32 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−113.0–−111.6 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−2.3540–−2.3550 MJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H224, H315, H319, H335 | ||
| Precautionary statements | P210, P261, P305+P351+P338 | ||
| Flash point | −18 °C (0 °F; 255 K) | ||
| Autoignition temperature |
402 °C (756 °F; 675 K) | ||
| Explosive limits | 2–10.4% | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
| ||
| LC50 (median concentration) | 4,000 ppm (rat, 4 hr) | ||
| LCLo (lowest published) | 7000 ppm (mouse, 40 min) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 5 ppm (12 mg/m) | ||
| REL (Recommended) | None established | ||
| IDLH (Immediate danger) | 750 ppm | ||
| Related compounds | |||
| Related alkanamines | |||
| Related compounds | 2-Methyl-2-nitrosopropane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Isopropylamine (also known as monoisopropyl amine, MIPA, or 2-propylamine) is an organic compound, an amine. It is a hygroscopic colorless liquid with ammonia-like odor. It is miscible with water and flammable. It is a valuable intermediate in chemical industry.
Reactions
Isopropylamine exhibits reactions typical of other simple alkyl amines, i.e. protonation, alkylation, acylation, condensation with carbonyls. Like other simple aliphatic amines, isopropylamine is a weak base: the pKa of is 10.63.
Preparation and use
Isopropylamine can be obtained by reaction of isopropyl alcohol with ammonia in presence of a catalyst:
- (CH3)2CHOH + NH3 → (CH3)2CHNH2 + H2O
Isopropylamine is a building block for the preparation of many herbicides and pesticides including atrazine, bentazon, glyphosate, imazapyr, ametryne, desmetryn, prometryn, pramitol, dipropetryn, propazine, fenamiphos, and iprodione. It is a regulating agent for plastics, an intermediate in organic synthesis of coating materials, plastics, pesticides, rubber chemicals, pharmaceuticals and others, and is an additive in the petroleum industry.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0360". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Isopropylamine". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 14 April 2015.
- ^ Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
{{cite book}}: CS1 maint: multiple names: authors list (link) - H. K. Hall, Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
External links
- International Chemical Safety Card 0908
- NIOSH Pocket Guide to Chemical Hazards. "#0360". National Institute for Occupational Safety and Health (NIOSH).