| |
| Names | |
|---|---|
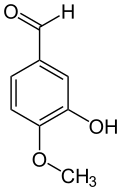
| Preferred IUPAC name 3-Hydroxy-4-methoxybenzaldehyde | |
| Other names
5-Formylguaiacol 3-Hydroxy-p-anisaldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1073021 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.724 |
| EC Number |
|
| MeSH | Isovanillin |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H8O3 |
| Molar mass | 152.149 g·mol |
| Appearance | Translucent crystals |
| Melting point | 113 to 116 °C (235 to 241 °F; 386 to 389 K) |
| Boiling point | 179 °C (354 °F; 452 K) at 15 mmHg |
| log P | 1.25 |
| Acidity (pKa) | 9.248 |
| Related compounds | |
| Related compounds | Anisaldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Isovanillin is a phenolic aldehyde, an organic compound and isomer of vanillin. It is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid, which could make it a candidate drug for use in alcohol aversion therapy. Isovanillin can be used as a precursor in the chemical total synthesis of morphine. The proposed metabolism of isovanillin (and vanillin) in rat has been described in literature, and is part of the WikiPathways machine readable pathway collection.
See also
References
- "Isovanillin". The PubChem Project. National Center for Biotechnology Information.
- "isovanillin - Compound Summary (CID 12127)".
- Georgios Panoutsopoulos; Christine Beedham (2005). "Enzymatic Oxidation of Vanillin, Isovanillin and Protocatechuic Aldehyde with Freshly Prepared Guinea Pig Liver Slices". Cell Physiol Biochem. 15 (1–4): 89–98. arXiv:quant-ph/0403227. doi:10.1159/000083641. PMID 15665519. S2CID 17057295.
- Uchida, Kenji; Yokoshima, Satoshi; Kan, Toshiyuki; Fukuyama, Tohru (2006). "Total Synthesis of (±)-Morphine". Organic Letters. 8 (23): 5311–5313. doi:10.1021/ol062112m. PMID 17078705.
- Uchida, Kenji; Yokoshima, Satoshi; Kan, Toshiyuki; Fukuyama, Tohru (2009). "Total Synthesis of (±)-Morphine". Heterocycles. 77 (2): 1219–1234. doi:10.1021/ol062112m. PMID 17078705. Retrieved 27 December 2013.
- Strand, L. P.; Scheline, R. R. (January 1975). "The metabolism of vanillin and isovanillin in the rat". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 5 (1): 49–63. doi:10.3109/00498257509056093. ISSN 0049-8254. PMID 1154798.
- "Vanillin and isovanillin metabolism". WikiPathways. 2019-10-31.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |