| |
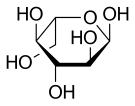 Haworth projection of α-l-glucopyranose | |
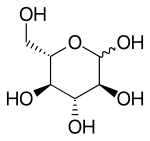
 Fischer projection of l-glucose | |
| Names | |
|---|---|
| IUPAC name l-Glucose | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | L-Glc |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H12O6 |
| Molar mass | 180.156 g·mol |
| Density | 1.54 g/cm |
| Solubility in water | 91 g/100 mL |
| Hazards | |
| Safety data sheet (SDS) | ICSC 0865 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
l-Glucose is an organic compound with formula C6H12O6 or O=CH5H, specifically one of the aldohexose monosaccharides. As the l-isomer of glucose, it is the enantiomer of the more common d-glucose.
l-Glucose does not occur naturally in living organisms, but can be synthesized in the laboratory. l-Glucose is indistinguishable in taste from d-glucose, but cannot be used by living organisms as a source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway. One of the known exceptions is in Burkholderia caryophylli, a plant pathogenic bacterium, which contains the enzyme d-threo-aldose 1-dehydrogenase which is capable of oxidizing l-glucose.
Like the d-isomer, l-glucose usually occurs as one of four cyclic structural isomers—α- and β-l-glucopyranose (the most common, with a six-atom ring), and α- and β-l-glucofuranose (with a five-atom ring). In water solution, these isomers interconvert in matters of hours, with the open-chain form as an intermediate stage.
Uses
l-Glucose was once proposed as a low-calorie sweetener and it is suitable for patients with diabetes mellitus, but it was never marketed due to excessive manufacturing costs.
The acetate derivative of l-glucose, l-glucose pentaacetate, was found to stimulate insulin release, and might therefore be of therapeutic value for type 2 diabetes. l-Glucose was also found to be a laxative, and has been proposed as a colon-cleansing agent which would not produce the disruption of fluid and electrolyte levels associated with the significant liquid quantities of bad-tasting osmotic laxatives conventionally used in preparation for colonoscopy.
References
- ^ A Natural Way to Stay Sweet, NASA, retrieved 2009-09-02.
- Sasajima, K.; Sinskey, A. (1979). "Oxidation of l-glucose by a Pseudomonad". Biochimica et Biophysica Acta (BBA) - Enzymology. 571 (1): 120–126. doi:10.1016/0005-2744(79)90232-8. PMID 40609.
- Malaisse, W. J. (1998), "The riddle of L-glucose pentaacetate insulinotropic action", Int. J. Mol. Med., 2 (4): 383–88, doi:10.3892/ijmm.2.4.383, PMID 9857221, archived from the original on 2011-07-16.
- Raymer, Geoffrey S.; Hartman, Donald E.; Rowe, William A.; Werkman, Robert F.; Koch, Kenneth L. (2003), "An open-label trial of L-glucose as a colon-cleansing agent before colonoscopy", Gastrointest. Endosc., 58 (1): 30–35, doi:10.1067/mge.2003.293, PMID 12838217
External links
 Media related to L-Glucose at Wikimedia Commons
Media related to L-Glucose at Wikimedia Commons