| |
| Names | |
|---|---|
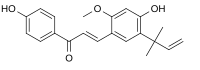
| Preferred IUPAC name (2E)-3--1-(4-hydroxyphenyl)prop-2-en-1-one | |
| Other names Licochalcone a | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.163.544 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H22O4 |
| Molar mass | 338.403 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P264, P280 |
| Safety data sheet (SDS) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Licochalcone A is a chalconoid, a type of natural phenol. It can be isolated from the root of Glycyrrhiza glabra (liquorice) or Glycyrrhiza inflata. It shows antimalarial, anticancer, antibacterial and antiviral (specifically against influenza neuraminidase) properties in vitro.
References
- ^ "Licochalcone A Safety Data Sheet" (PDF). Cayman Chemicals.
- ^ Fu, Y.; Hsieh, T. C.; Guo, J.; Kunicki, J.; Lee, M. Y. W. T.; Darzynkiewicz, Z.; Wu, J. M. (2004). "Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells". Biochemical and Biophysical Research Communications. 322 (1): 263–270. doi:10.1016/j.bbrc.2004.07.094. PMID 15313200.
- ^ Friis-Møller, A.; Chen, M.; Fuursted, K.; Christensen, S. R. B. G.; Kharazmi, A. (2002). "In Vitro Antimycobacterial and Antilegionella Activity of Licochalcone a from Chinese Licorice Roots". Planta Medica. 68 (5): 416–419. doi:10.1055/s-2002-32087. PMID 12058317.
- Chen, M.; Theander, T. G.; Christensen, S. B.; Hviid, L.; Zhai, L.; Kharazmi, A. (1994). "Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. Yoelii infection". Antimicrobial Agents and Chemotherapy. 38 (7): 1470–1475. doi:10.1128/aac.38.7.1470. PMC 284578. PMID 7979274.
- Dao, TT; Nguyen, PH; Lee, HS; Kim, E; Park, J; Lim, SI; Oh, WK (January 2011). "Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata". Bioorganic & Medicinal Chemistry Letters. 21 (1): 294–8. doi:10.1016/j.bmcl.2010.11.016. PMID 21123068.
| Chalconoids and their glycosides | |
|---|---|
| Chalconoids | |
| Chalconoid glycosides | |
| Acylated chalconoids |
|
| O-methylated chalconoids |
|
| Flavokavains | |
| Synthetic | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |