| |
| Names | |
|---|---|
| Preferred IUPAC name Methoxymethanol | |
| Other names Formaldehyde methyl hemiacetal | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1900186 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.022.476 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H6O2 |
| Molar mass | 62.068 g·mol |
| Density | 0.948 |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Warning |
| Hazard statements | H226, H302, H371 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P260, P264, P270, P280, P301+P312, P303+P361+P353, P309+P311, P330, P370+P378, P403+P235, P405, P501 |
| Flash point | 39.9 °C (103.8 °F; 313.0 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
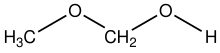
Methoxymethanol is a chemical compound which is both an ether and an alcohol, a hemiformal. The structural formula can be written as CH3OCH2OH. It has been discovered in space.
Formation
Methoxymethanol forms spontaneously when a water solution of formaldehyde and methanol are mixed. or when formaldehyde is bubbled through methanol.
In space methoxymethanol can form when methanol radicals (CH2OH or CH3O) react. These are radiolysis products derived when ultraviolet light or cosmic rays hit frozen methanol.
Methanol can react with carbon dioxide and hydrogen at 80°C and some pressure with a ruthenium or cobalt catalyst, to yield some methoxymethanol.
Properties
Different conformations of the molecule are Gauche-gauce (Gg), Gauche-gauce' (Gg'), and Trans-gauche (Tg).
References
- ^ Maiwald, Michael; Fischer, Holger H.; Ott, Michael; Peschla, Roger; Kuhnert, Christian; Kreiter, Cornelius G.; Maurer, Gerd; Hasse, Hans (January 2003). "Quantitative NMR Spectroscopy of Complex Liquid Mixtures: Methods and Results for Chemical Equilibria in Formaldehyde−Water−Methanol at Temperatures up to 383 K". Industrial & Engineering Chemistry Research. 42 (2): 259–266. doi:10.1021/ie0203072.
- McGuire, Brett A.; Shingledecker, Christopher N.; Willis, Eric R.; Burkhardt, Andrew M.; El-Abd, Samer; Motiyenko, Roman A.; Brogan, Crystal L.; Hunter, Todd R.; Margulès, Laurent; Guillemin, Jean-Claude; Garrod, Robin T.; Herbst, Eric; Remijan, Anthony J. (2017). "ALMA Detection of Interstellar Methoxymethanol (CH3OCH2OH)". The Astrophysical Journal. 851 (2): L46. arXiv:1712.03256. Bibcode:2017ApJ...851L..46M. doi:10.3847/2041-8213/aaa0c3. S2CID 119211919.
- ^ Hays, Brian M.; Widicus Weaver, Susanna L. (6 May 2013). "Theoretical Examination of O(D) Insertion Reactions to Form Methanediol, Methoxymethanol, and Aminomethanol". The Journal of Physical Chemistry A. 117 (32): 7142–7148. Bibcode:2013JPCA..117.7142H. doi:10.1021/jp400753r. PMID 23646865.
- Celik, Fuat E.; Lawrence, Henry; Bell, Alexis T. (June 2008). "Synthesis of precursors to ethylene glycol from formaldehyde and methyl formate catalyzed by heteropoly acids". Journal of Molecular Catalysis A: Chemical. 288 (1–2): 87–96. doi:10.1016/j.molcata.2008.03.029.
- Dixneuf, Pierre H.; Soulé, Jean-François (2019). Organometallics for Green Catalysis. Springer. pp. 69–70. ISBN 978-3-030-10955-4.
- Motiyenko, R. A. (21 June 2016). "Millimeter-wave spectroscopy of methoxymethanol". 71st International Symposium on Molecular Spectroscopy: 1. Bibcode:2016isms.confETH04M. doi:10.15278/isms.2016.TH04. hdl:2142/91121. ISBN 978-1-5330-5390-9.