| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1-Ethenylpyrrolidin-2-one | |||
| Other names
1-Vinylpyrrolidin-2-one 1-Ethenyl-2-pyrrolidone N-Ethenyl-2-pyrrolidone N-Vinyl-2-pyrrolidone 1-Vinyl-2-pyrrolidone N-Vinylbutyrolactam | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.637 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H9NO | ||
| Molar mass | 111.144 g·mol | ||
| Density | 1.04 g/cm | ||
| Melting point | 13–14 °C (55–57 °F; 286–287 K) | ||
| Boiling point | 92–95 °C (198–203 °F; 365–368 K) 11 mmHg | ||
| Vapor pressure | 0.1 mmHg (24 °C) | ||
| Refractive index (nD) | 1.512 | ||
| Hazards | |||
| Flash point | 95 °C (203 °F; 368 K) | ||
| Autoignition temperature |
685 °C (1,265 °F; 958 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
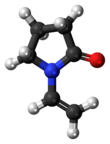
N-Vinylpyrrolidone (NVP) is an organic compound consisting of a 5-membered lactam ring linked to a (2 carbon) vinyl group. It is a colorless liquid although commercial samples can appear yellowish.
It is produced industrially by vinylation of 2-pyrrolidone, i.e. the base-catalyzed reaction with acetylene. It is the precursor to polyvinylpyrrolidone (PVP), an important synthetic material. The NVP monomer is commonly used as a reactive diluent in ultraviolet and electron-beam curable polymers applied as inks, coatings or adhesives.
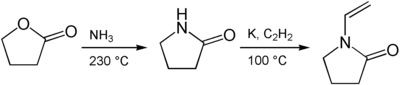
Synthesis
Starting from γ-Butyrolactone, 2-pyrrolidone is synthesized by treatment with ammonia. Subsequently, acetylene is used to introduce the vinyl group.
See also
- Methylpyrrolidone (NMP)
- 2-Pyrrolidone (2-Py)
References
- ^ "1-Vinyl-2-pyrrolidinone". Sigma-Aldrich.
- ^ Harreus, Albrecht Ludwig; Backes, R.; Eichler, J.-O.; Feuerhake, R.; Jäkel, C.; Mahn, U.; Pinkos, R.; Vogelsang"2-Pyrrolidone, R. (2011). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_457.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: numeric names: authors list (link) - Teodorescu, Mirela; Bercea, Maria (23 June 2015). "Poly(vinylpyrrolidone) – A Versatile Polymer for Biomedical and Beyond Medical Applications". Polymer-Plastics Technology and Engineering. 54 (9): 923–943. doi:10.1080/03602559.2014.979506.
- Pässler, Peter; Hefner, Werner; Buckl, Klaus; Meinass, Helmut; Meiswinkel, Andreas; Wernicke, Hans-Jürgen; Ebersberg, Günter; Müller, Richard; Bässler, Jürgen; Behringer, Hartmut; Mayer, Dieter (2008). "Acetylene Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_097.pub3. ISBN 3527306730.