| |
| Names | |
|---|---|
| IUPAC name Trimethylvinylammonium hydroxide | |
| Other names Vitaloid; N,N,N-Trimethylethenaminium hydroxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.006.678 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H13NO |
| Molar mass | 103.16 |
| Appearance | Syrupy liquid |
| Solubility in water | Soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Neurine is an alkaloid found in egg yolk, brain, bile and in cadavers. It is formed during putrefaction of biological tissues by the dehydration of choline. It is a poisonous, syrupy liquid with a fishy odor.
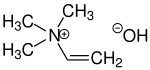
Neurine is a quaternary ammonium salt with three methyl groups and one vinyl group attached to the nitrogen atom. Synthetically, neurine can be prepared by the reaction of acetylene with trimethylamine. Neurine is unstable and decomposes readily to form trimethylamine.
References
- Gardner, C.; Kerrigan, V.; Rose, J. D.; Weedon, B. C. L. (1949-01-01). "169. Acetylene reactions. Part IV. Formation of trimethylvinyl- and tetramethyl-ammonium hydroxide from acetylene and aqueous trimethylamine". Journal of the Chemical Society (Resumed): 789–792. doi:10.1039/JR9490000789. ISSN 0368-1769.
- Merck Index, 11th Edition, 6393.