| |
| Names | |
|---|---|
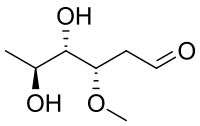
| IUPAC name (3S,4S,5S)-4,5-Dihydroxy-3-methoxyhexanal | |
| Other names 2,6-Dideoxy-3-O-methyl-L-arabinohexose | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| KEGG | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H14O4 |
| Molar mass | 162.185 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Oleandrose is a type of carbohydrate with the chemical formula C7H14O4. With a six-carbon chain, it is classified as a hexose. With two hydroxyl groups replaced with hydrogen atoms, it is a dideoxy sugar. The hydroxyl group at C3 is methylated.
Occurrence
Oleandrdose is found in the leaves of Nerium oleander and may contribute to the toxicity of the plant. Oleandrose is also a component of several naturally-occurring chemical compounds including the avermectins (emamectin, abamectins, ivermectin, and others), the macrolide antibiotic oleandomycin, and the cardiac glycoside oleandrin.
Laboratory syntheses of L-oleandrose and DL-oleandrose have been reported.
See also
- Sarmentose, a diastereomeric dideoxy sugar
References
- Siddiqui, Bina Shaheen; Khatoon, Nasima; Begum, Sabira; Farooq, Ahsana Dar; Qamar, Kehkashan; Bhatti, Huma Aslam; Ali, Syed Kashif (2012). "Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity". Phytochemistry. 77: 238–244. Bibcode:2012PChem..77..238S. doi:10.1016/j.phytochem.2012.01.001. PMID 22281382.
- Bakir Çilesizoğlu, Neşe; Yalçin, Emine; Çavuşoğlu, Kültiğin; Sipahi Kuloğlu, Selin (2022). "Qualitative and quantitative phytochemical screening of Nerium oleander L. Extracts associated with toxicity profile". Scientific Reports. 12 (1): 21421. Bibcode:2022NatSR..1221421B. doi:10.1038/s41598-022-26087-0. PMC 9742154. PMID 36504046.
- Bredenkamp, Martin W.; Holzapfel, Cedric W.; Toerien, Francois (1992). "Alternative Syntheses of L-(-)-Oleandrose from L-RhamnosePreparation of Glycals". Synthetic Communications. 22 (17): 2459–2477. doi:10.1080/00397919208021642.
- Berti, G.; Catelani, G.; Colonna, F.; Monti, L. (1982). "A highly diastereoselective synthesis of dl-oleandrose". Tetrahedron. 38 (20): 3067–3072. doi:10.1016/0040-4020(82)80194-4.