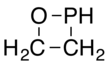| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
1,2-Oxaphosphetane 1,3-Oxaphosphetane | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChemSpider |
| ||
| PubChem CID | |||
| CompTox Dashboard (EPA) |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C2H5OP | ||
| Molar mass | 76.035 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||

An oxaphosphetane is a molecule containing a four-membered ring with one phosphorus, one oxygen and two carbon atoms. In a 1,2-oxaphosphetane phosphorus is bonded directly to oxygen, whereas a 1,3-oxaphosphetane has the phosphorus and oxygen atoms at opposite corners.
1,2-Oxaphosphetanes are rarely isolated but are important intermediates in the Wittig reaction and related reactions such as the Seyferth–Gilbert homologation and the Horner–Wadsworth–Emmons reaction. Edwin Vedejs's NMR studies first revealed the importance of oxaphosphetanes in the mechanism of the Wittig reaction in the 1970s.
In 2005 the first isolation of 1,2-Oxaphosphetanes (typical Wittig intermediates) was reported. One of the compounds was characterized by X-ray crystallography and NMR. Although relatively stable, thermal decomposition of these oxaphosphetanes gave a phosphonium salt, which slowly dissociated to the Wittig reaction starting materials, the carbonyl and olefin compounds.
References
- M. Hamaguchi; Y. Iyamaa; E. Mochizukia; T. Oshima (2005). "First isolation and characterization of 1,2-oxaphosphetanes with three phenyl groups at the phosphorus atom in typical Wittig reaction using cyclopropylidenetriphenylphosphorane". Tetrahedron Letters. 46 (51): 8949–8952. doi:10.1016/j.tetlet.2005.10.086.
- Byrne, Peter A.; Gilheany, Declan G. (2013). "The modern interpretation of the Wittig reaction mechanism". Chemical Society Reviews. 42 (16): 6670–6696. doi:10.1039/C3CS60105F. hdl:10197/4939. PMID 23673458.
- Vedejs E (30 July 2004). "Studies in Heteroelement-Based Synthesis". The Journal of Organic Chemistry. 69 (16): 5159–5167. doi:10.1021/jo049360l. PMID 15287757.
- "Memorial Resolution of the Faculty of the University of Wisconsin-Madison" (PDF). University of Wisconsin-Madison. Archived from the original (PDF) on 9 May 2020. Retrieved 9 May 2020.
- M. Hamaguchi; Y. Iyamaa; E. Mochizukia; T. Oshima (2005). "First isolation and characterization of 1,2-oxaphosphetanes with three phenyl groups at the phosphorus atom in typical Wittig reaction using cyclopropylidenetriphenylphosphorane". Tetrahedron Letters. 46 (51): 8949–8952. doi:10.1016/j.tetlet.2005.10.086.
This article about a heterocyclic compound is a stub. You can help Misplaced Pages by expanding it. |