| This article may be in need of reorganization to comply with Misplaced Pages's layout guidelines. Please help by editing the article to make improvements to the overall structure. (December 2021) (Learn how and when to remove this message) |
This page provides supplementary chemical data on p-xylene.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.4958 at 20 °C |
| Dielectric constant, εr | 2.2 ε0 at 20 °C |
| Surface tension | 29.92 dyn/cm at 5 °C 28.27 dyn/cm at 20 °C 24.2 dyn/cm at 60 °C |
| Viscosity | 0.7385 mPa·s at 10 °C 0.6475 mPa·s at 20 °C 0.5134 mPa·s at 40 °C 0.3519 mPa·s at 80 °C 0.2424 mPa·s at 130 °C |
| Solubility | 0.160 g/L at 0 °C 0.181 g/L at 25 °C 0.22 g/L at 40 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 286.3 K (13.15 °C), ? Pa |
| Critical point | 617 K (344 °C), 3500 kPa |
| Std enthalpy change of fusion, ΔfusH |
17.1 kJ/mol |
| Std entropy change of fusion, ΔfusS |
59.8 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
35.7 kJ/mol at 138 °C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfHsolid |
? kJ/mol |
| Standard molar entropy, Ssolid |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfHliquid |
−24.4 kJ/mol |
| Standard molar entropy, Sliquid |
247 J/(mol K) |
| Enthalpy of combustion, ΔcH | −4552 kJ/mol |
| Heat capacity, cp | 181.7 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfHgas |
1.796000E+04 kJ/kg-mol at 25 °C |
| Standard molar entropy, Sgas |
? J/(mol K) |
| Heat capacity, cp | 163.2 J/(mol K) at 120 °C |
| van der Waals' constants | a = 3134 L kPa/mol b = 0.1809 liter per mole |
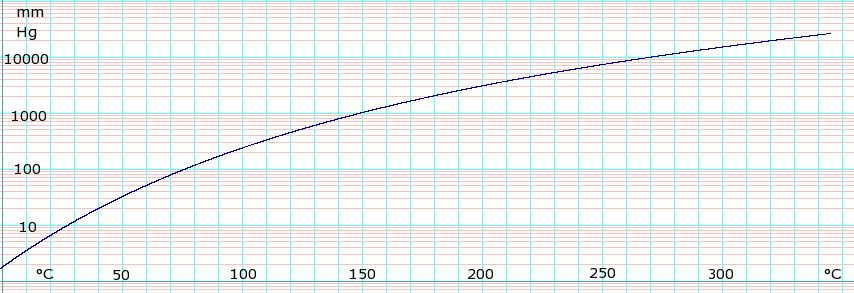
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | −8.1 | 27.3 | 54.4 | 75.9 | 115.9 | 138.3 | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Distillation data
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |
|---|---|
| Spectrum | |
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Spectrum | NIST |
| Major absorption bands | 793.94 cm |
| NMR | |
| Spectrum | AIST |
| Proton NMR | 2.296, 7.046 |
| Carbon-13 NMR | 134.66, 128.97, 20.90 |
| MS | |
| Spectrum | NIST |
| Masses of main fragments |
106, 91, 77 |
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
- Notes
- Lange's Handbook of Chemistry, 10th ed, pp 1661–1663
- Lange's Handbook of Chemistry, 10th ed, pp 1669–1674
- CRC Handbook of Chemistry and Physics, 85th ed. p8-111
- ^ "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 26 May 2007.
- Lange's Handbook of Chemistry 10th ed, pp 1522–1524
- ^ "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 26 May 2007.
- Bibliography
- NIST Standard Reference Database
- "University of Akron, Chemistry Department, Chemical Database". Archived from the original on 13 January 2004. Retrieved 1 February 2007.
- "National Institute of Advanced Industrial Science and Technology". Archived from the original on 5 May 2006. Retrieved 1 February 2007.

 obtained from CHERIC
obtained from CHERIC