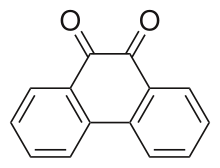
| |
| Names | |
|---|---|
| Preferred IUPAC name Phenanthrene-9,10-dione | |
| Other names 9,10-Phenanthrenequinone | |
| Identifiers | |
| CAS Number | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.377 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | C14H8O2 |
| Molar mass | 208.216 g·mol |
| Appearance | Orange solid |
| Odor | Odorless |
| Melting point | 209 °C (408 °F; 482 K) |
| Boiling point | 360 °C (680 °F; 633 K) |
| Solubility in water | Slightly soluble (7.5 mg L) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H315, H319, H400 |
| Precautionary statements | P264, P273, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362, P391, P501 |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Phenanthrenedione is a quinone derivative of a polycyclic aromatic hydrocarbon. It is an orange, water-insoluble solid.
Laboratory synthesis and use
It has been prepared by oxidation of phenanthrene with chromic acid.
It is used as an artificial mediator for electron acceptor/donor in Mo/W containing formate dehydrogenase reduction of carbon dioxide to formate and vice versa. It is a better electron acceptor than the natural nicotinamide adenine dinucleotide (NAD).
Safety
It is cytotoxic and potentially mutagenic.
Phenanthrenequinone is one of many contributors to harmful particulate emissions from diesel motor vehicles.
Related compounds
References
- " 84-11-7|Phenanthrenequinone|Toxnet|". nih.gov.
- Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2000). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227. ISBN 978-3527306732.
- Wendland, Ray; LaLonde, John (1954). "Phenanthrenequinone". Org. Synth. 34: 76. doi:10.15227/orgsyn.034.0076.
- Robert A. Kanaly; Natsuko Hamamura (September 2013). "9,10-Phenanthrenedione biodegradation by a soil bacterium and identification of transformation products by LC/ESI-MS/MS". Chemosphere. 92 (11): 1442–1449. Bibcode:2013Chmsp..92.1442K. doi:10.1016/j.chemosphere.2013.03.054. PMID 23611246.
- Durant, John L.; Busby, William F.; Lafleur, Arthur L.; Penman, Bruce W.; Crespi, Charles L. (1996). "Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols". Mutation Research/Genetic Toxicology. 371 (3–4): 123–157. doi:10.1016/s0165-1218(96)90103-2. PMID 9008716.
- Rogge, Wolfgang F.; Hildemann, Lynn M.; Mazurek, Monica A.; Cass, Glen R.; Simoneit, Bernd R. T. (1993). "Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks". Environmental Science & Technology. 27 (4): 636–651. doi:10.1021/es00041a007.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |