| |
| Names | |
|---|---|
| Preferred IUPAC name 2,6-Dimethylhepta-2,5-dien-4-one | |
| Other names
Phorone Diisopropylidene acetone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.261 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1993 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
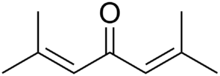
| Chemical formula | ((CH3)2C=CH)2C=O |
| Molar mass | 138.210 g·mol |
| Appearance | Yellow crystals |
| Odor | Geranium |
| Density | 0.885 g/cm |
| Melting point | 28 °C (82 °F; 301 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| Hazards | |
| Flash point | 79 °C (174 °F; 352 K) |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula C9H14O or ((CH3)2C=CH)2C=O.
Preparation
It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone". On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid.
- CaC10H14O4 → C9H14O + CaCO3
It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated.
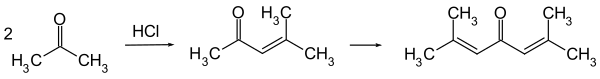
Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble.
Reactions
Phorone can condense with ammonia to form triacetone amine.
See also
References
- Merck Index, 11th Edition, 7307.
- Laurent, Auguste (1837). "Sur les acides pinique et sylvique, et sur le camphoryle" [On pinic and sylvic acids, and on camphoryl]. Annales de Chimie et de Physique. 2nd series (in French). 65: 324–332.; see "Camphoryle", pp. 329–330.
- See:
- Gerhardt, Charles (1849) Comptes rendus des travaux de chimie (Paris, France: Masson, 1849), p. 385. (in French)
- Gerhardt; Liès-Bodart (1849). "Trockne Destillation des camphorsauren Kalks" [Dry distillation of calcium camphorate]. Annalen der Chemie und Pharmacie (in German). 72 (3): 293–294. doi:10.1002/jlac.18490720327. From p. 293: "Dieses Oel, welches Gerhardt und Lies-Bodart mit dem Namen Phoron bezeichnen, … " (This oil, which Gerhardt and Liès-Bodart designate by the name "phorone", … )
- Watts, Henry, A Dictionary of Chemistry and the Allied Branches of Other Sciences (London, England: Longmans, Green, and Co., 1863), vol. 1, "Camphorone", p. 733.
- Kekulé, August (1866). Lehrbuch der organischen Chemie [Textbook of organic chemistry] (in German). Vol. 2nd vol. Erlangen, (Germany): Ferdinand Enke. p. 463.
- Hardo Siegel; Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 978-3-527-30673-2.