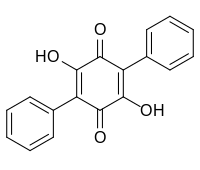
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2-Dihydroxy-2,2-dione | |
| Other names Polyporin; Orygameic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| MeSH | C118527 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H12O4 |
| Molar mass | 292.290 g·mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Toxic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Polyporic acid is a para-terphenyl benzoquinone compound first identified by German chemist Stahlschmidt from a mycelial culture of the fungus species Hapalopilus nidulans in 1877. This chemical, present at 20–40% of the fresh weight of the fruit bodies, inhibits the enzyme dihydroorotate dehydrogenase. It is found in other mushrooms, but in much lower amounts.
In animal studies, consumption of polyporic acid caused reduced locomotor activity, depressed visual placing response, hepatorenal failure, metabolic acidosis, hypokalaemia, and hypocalcaemia. Because these effects are similar to those observed in individuals poisoned by H. nidulans, polyporic acid is thought to be the primary toxin in H. nidulans.
Polyporic acid has some antifungal and antibacterial activity. It has been shown to be an intermediate in the biosynthesis of allantofuranone, a gamma-lactone antibiotic from the fungus Allantophomopsis lycopodina.
References
- Stahlschmidt C. (1877). "Ueber eine neue in der Natur vorkommende organische Säure" [A new naturally occurring organic acid]. Justus Liebigs Annalen der Chemie. 187 (2–3): 177–197. doi:10.1002/jlac.18771870204.
- Spatafora C, Calì V, Tringali C (2003). Polyhydroxy-p-terphenyls and related p-terphenylquinones from fungi: overview and biological properties. Vol. 29. pp. 263–307. doi:10.1016/S1572-5995(03)80009-1. ISBN 9780444515100.
{{cite book}}:|journal=ignored (help) - Räisänen R. (2009). "Dyes from lichens and mushrooms". In Bechtold T, Mussak R (eds.). Handbook of Natural Colorants. Chichester, UK: John Wiley & Sons. p. 192. ISBN 978-0-470-74496-3.
- ^ Kraft J, Bauer S, Keilhoff G, Miersch J, Wend D, Riemann D, Hirschelmann R, Holzhausen HJ, Langner J (1998). "Biological effects of the dihydroorotate dehydrogenase inhibitor polyporic acid, a toxic constituent of the mushroom Hapalopilus rutilans, in rats and humans". Archives of Toxicology. 72 (11): 711–721. doi:10.1007/s002040050565. PMID 9879809. S2CID 41488737.
- Brewer D, Maass WS, Taylor A (1977). "The effect on fungal growth of some 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology. 23 (7): 845–51. doi:10.1139/m77-126. PMID 884625.
- Brewer D, Jen WC, Jones GA, Taylor A (1984). "The antibacterial activity of some naturally occurring 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology. 30 (8): 1068–1092. doi:10.1139/m84-166. PMID 6541963.
- Schüffler A, Liermann JC, Opatz T, Anke T (2011). "Elucidation of the biosynthesis and degradation of allantofuranone by isotopic labelling and fermentation of modified precursors". ChemBioChem. 12 (1): 148–154. doi:10.1002/cbic.201000448. PMID 21181846. S2CID 30217850.