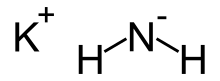
| |

| |
| Names | |
|---|---|
| IUPAC name Potassium amide | |
| Other names Potassamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.037.508 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | KNH2 |
| Molar mass | 55.121 g·mol |
| Appearance | white solid |
| Odor | ammonia-like |
| Density | 1.57 g/cm |
| Melting point | 338 °C (640 °F; 611 K) |
| Solubility in water | reacts |
| Solubility | ammonia: 3.6 g/(100 mL) |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH298) |
-128.9 kJ/mol |
| Related compounds | |
| Other cations | Lithium amide Sodium amide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.
Production
Potassium amide is produced by the reaction of ammonia with potassium. The reaction typically requires a catalyst.
Structure
Traditionally KNH2 is viewed as a simple salt, but it has significant covalent character and is highly aggregated in ammonia solution. The compound has been characterized by X-ray crystallography as the solvent-free form as well as the mono- and diammonia solvates. In KNH2·2NH3, the potassium centers are each bonded to two amido ligands and four ammonia ligands, all six of which bridge to adjacent potassium centers. The result is a chain of hexacoordinate potassium ions. The K–NH2 distances are 2.7652(11) whereas the K–NH3 distances are respectively 2.9234(11) and 3.0698(11) Å.
References
- Takaki, Katherine S. (2001). "Potassium Amide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp193. ISBN 0471936235.
- O. Glemser, H. Sauer (1963). "Silver Amide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. p. 1043.
- Juza, R.; Jacobs, H.; Klose, W. (1965). "Die Kristallstrukturen der Tieftemperaturmodifikationen von Kalium- und Rubidiumamid". Zeitschrift für Anorganische und Allgemeine Chemie. 338 (3–4): 171–178. doi:10.1002/zaac.19653380309.
- Kraus, Florian; Korber, Nikolaus (2005). "Hydrogen Bonds in Potassium Amide-Ammonia(1/2), KNH22NH3". Zeitschrift für Anorganische und Allgemeine Chemie. 631 (6–7): 1032–1034. doi:10.1002/zaac.200400467.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |