| |
| Names | |
|---|---|
| IUPAC name 3,3′,3′′,3′′′-tetrapropanoic acid | |
| Systematic IUPAC name 3,3′,3′′,3′′′-tetrapropanoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1209089 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | hydroxymethylbilane |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C40H46N4O17 |
| Molar mass | 854.81 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
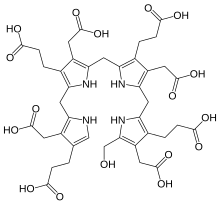
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB.
Structure
The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges −CH2−. The chain starts with a hydroxymethyl group −CH2−OH and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group −CH2−COOH and a propionic acid group −CH2−CH2−COOH, in that order.
Metabolism
HMB is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase:

The enzyme uroporphyrinogen III synthase closes the chain to form uroporphyrinogen III:

Uroporphyrinogen III is a porphyrinogen, which is a class of compounds with the hexahydroporphine macrocycle. In the absence of the enzyme, the compound undergoes spontaneous cyclization and becomes uroporphyrinogen I.
References
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- ^ Voet, Donald; Voet, Judith G. (2011). Biochemistry (4. ed.). Hoboken, NJ: Wiley. ISBN 978-0-470-57095-1.
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- Sassa, S.; Kappas, A. (2000). "Molecular aspects of the inherited porphyrias". Journal of Internal Medicine. 247 (2): 169–178. doi:10.1046/j.1365-2796.2000.00618.x. PMID 10692079. S2CID 36820694.
| Heme metabolic intermediates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Porphyrin biosynthesis |
| ||||||||||||
| Heme degradation and excretion |
| ||||||||||||