| |
| Names | |
|---|---|
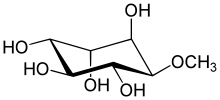
| IUPAC name (1R,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol | |
| Other names
Quebrachitol L-Quebrachitol (-)-Quebrachitol 2-O-methyl-l-inositol 2-0-methyl-chiro-inositol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H14O6 |
| Molar mass | 194.18 g/mol |
| Appearance | White to off-white powder |
| Melting point | 190 to 198 °C (374 to 388 °F; 463 to 471 K) |
| Solubility in water | Soluble in DMSO, dimethyl formamide, or water |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Quebrachitol is a naturally occurring optically active cyclitol, a cyclic polyol. It can be found in Allophylus edulis and in the serum left after the coagulation of the Hevea brasiliensis latex in the operation of rubber tapping. It is also found in Cannabis sativa, in Paullinia pinnata and in seabuckthorn.
It was first isolated by Tanret in 1887 from the bark of Aspidosperma quebracho. The substance was tested as a sweetening agent for diabetics in 1933. It shows a sweetening property half of that of sucrose but induces colic or diarrhoea at concentration used to render the food palatable.
Quebrachitol is a versatile building block in the construction of naturally occurring bioactive materials. For example, its conversion into antifungal (E)-β-methoxyacrylate, oudemansin X has been made.
References
- Díaz, Martina; González, Andrés; Castro-Gamboa, Ian; Gonzalez, David; Rossini, Carmen (13 October 2008). "First record of l-quebrachitol in Allophylus edulis (Sapindaceae)". Carbohydrate Research. 343 (15): 2699–2700. doi:10.1016/j.carres.2008.07.014. PMID 18715552.
- van Alphen, Jan (1951). "Quebrachitol". Industrial & Engineering Chemistry. 43: 141–145. doi:10.1021/ie50493a041.
- 1955 - ACTA UNIVERSITATIS PALACKIANAE OLOMUCENSIS - TOM. VI. - HEMP AS A MEDICAMENT, Properties of isolated substances. Prof. Jan Kabelik, A brief survey of the methods of isolation and the physical and chemical properties and structures of the isolated antibacterial substances. F. Santavy & Z. Krejci
- Some new data about antiviral and related activities of seabuckthorn principals and the prospects of their use. Shipulina L.D., All-Russian Research Institute of Medicinal and Aromatic Plants, Moscow, Russia
- McCance, RA; Lawrence, RD (1933). "An investigation of quebrachitol as a sweetening agent for diabetics". Biochem J. 27 (4): 986–9. doi:10.1042/bj0270986. PMC 1252976. PMID 16745234.
- Kiddle, James J. (1995). "Quebrachitol: A Versatile Building Block in the Construction of Naturally Occurring Bioactive Materials". Chemical Reviews. 95 (6): 2189–2202. doi:10.1021/cr00038a016.
- Total synthesis of antibiotic (−)-oudemansin X utilizing L-quebrachitol as a chiral pool. Chida N., Yamada K. and Ogawa S., Chemistry Letters, 1992, no4, pp. 687-690
External links
- "Quebrachitol on the Sigma-Aldrich website". Archived from the original on October 3, 2012. Retrieved September 19, 2016.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)