
| |
| Names | |
|---|---|
| Preferred IUPAC name N--4-(methanesulfonamido)benzamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Sematilide |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H23N3O3S |
| Molar mass | 313.42 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sematilide is an antiarrhythmic agent. It is the same structure as for procainamide, differing only by the placement of a mesyl sulfonamide moiety to the anilino nitrogen.
Synthesis
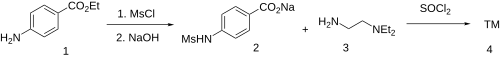
The reaction between Benzocaine (Ethyl 4-Aminobenzoate) (1) and mesyl chloride gives the sulfonamide, Ethyl 4-(Methylsulfonamido)benzoate . Base saponification followed by the removal of the water from the reaction mixture gives 4-benzoic acid sodium salt (2). Halogenation with thionyl chloride gives 4-Benzoyl Chloride . Amide formation with N,N-Diethylethylenediamine (3) then concludes the synthesis of Sematilide (4).
References
- Lumma, William C.; Wohl, Ronald A.; Davey, David D.; Argentieri, Thomas M.; DeVita, Robert J.; Gomez, Robert P.; Jain, Vijay K.; Marisca, Anthony J.; Morgan, Thomas K. (1987). "Rational design of 4-benzamides as class III antiarrhythmic agents". Journal of Medicinal Chemistry 30 (5): 755–758. doi:10.1021/jm00388a001.
- David D. Davey, William C. Lumma, Jr., Ronald A. Wohl, U.S. patent 4,544,654 (1985 to Schering A.G.).
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |