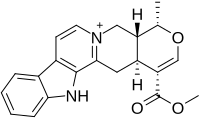
| |
| Names | |
|---|---|
| IUPAC name (19α)-16-(Methoxycarbonyl)-19-methyl-3,4,5,6,16,17-hexadehydro-18-oxayohimban-4-ium-1-ide | |
| Other names Methyl ester of serpentinic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.038.684 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H21N2O3 |
| Molar mass | 349.410 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Serpentine is a terpene indole alkaloid produced by several members of the family Apocynaceae (thus an "apocynaceae alkaloid"), including Catharanthus roseus and Rauvolfia serpentina.
See also
References
- Monforte-González, M; Ayora-Talavera, T; Maldonado-Mendoza, I. E; Loyola-Vargas, V. M (1992). "Quantitative analysis of serpentine and ajmalicine in plant tissues of Catharanthus roseus and hyoscyamine and scopolamine in root tissues of Datura stramonium by thin layer chromatography-densitometry". Phytochemical Analysis. 3 (3): 117. Bibcode:1992PChAn...3..117M. doi:10.1002/pca.2800030305.
- Leete, Edward (1961). "Biogenesis of the Rauwolfia alkaloids alkaloids—II". Tetrahedron. 14 (1–2): 35–41. doi:10.1016/0040-4020(61)80084-7.
This article about an alkaloid is a stub. You can help Misplaced Pages by expanding it. |