| |
| Names | |
|---|---|
| Other names silver; 1-hydroxy-1-oxopropan-2-olate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.036.221 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
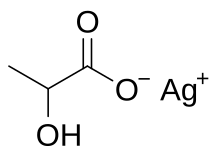
| Chemical formula | CH3CH(OH)COOAg |
| Molar mass | 196.93 g/mol |
| Appearance | Gray to purple powder or flakes |
| Melting point | 120–122 °C (248–252 °F; 393–395 K) |
| Boiling point | 227.6 °C (441.7 °F; 500.8 K) |
| Solubility in water | Soluble |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P302, P305, P338, P351, P352 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Silver lactate is an organic chemical compound, a salt of silver and lactic acid with the formula CH3CH(OH)COOAg.
Synthesis
Silver lactate can be made by the reaction of silver carbonate with lactic acid.
Physical properties
Silver lactate forms light gray crystals.
Silver lactate is soluble in water, slightly soluble in ethanol.
Silver lactate forms a crystalline hydrate of composition CH3CH(OH)COOAg•H2O.
Silver lactate is a reagent for the precipitation of uric acid.
Chemical properties
The compound reacts with triphenylphosphine gold chloride in a mixed solvent of benzene and dichloromethane to obtain colorless triphenylphosphine gold lactate.
The compound reacts with a tetraphosphine ligand, dppbpda, to obtain a coordination polymer n.
References
- Hacker, Gerhard W.; Gu, Jiang (17 April 2002). Gold and Silver Staining: Techniques in Molecular Morphology. CRC Press. p. 62. ISBN 978-1-4200-4023-4. Retrieved 18 January 2022.
- "Silver Lactate". American Elements. Retrieved 18 January 2022.
- "Silver lactate". Sigma Aldrich. Retrieved 18 January 2022.
- Hayat, M. A. (3 August 1995). Immunogold-Silver Staining: Principles, Methods, and Applications. CRC Press. p. 30. ISBN 978-0-8493-2449-9. Retrieved 18 January 2022.
- Cornell University Medical Bulletin. 1928. p. 296. Retrieved 18 January 2022.
- Fackler, John P.; Khan, M. Nazrul I.; King, Christopher; Staples, Richard J.; Winpenny, Richard E. P. (1 July 1991). "Decarboxylation of (triphenylphosphine)gold(I) carboxylates". Organometallics. 10 (7): 2178–2183. doi:10.1021/om00053a021. ISSN 0276-7333. Retrieved 23 January 2022.
- Zhang, Min; Feng, Meng-Yao; Yan, Jia-Jun; Li, Hai-Yan; Young, David James; Li, Hong-Xi; Ren, Zhi-Gang (21 June 2021). "New Silver(I)-P4 Coordination Polymers Strongly Adsorb Congo Red to Yield Composites with Enhanced Photocurrent Responses". European Journal of Inorganic Chemistry. 2021 (23): 2262–2265. doi:10.1002/ejic.202100228. S2CID 235558940. Retrieved 23 January 2022.
| Silver compounds | |||
|---|---|---|---|
| Silver(0,I) | |||
| Silver(I) |
| ||
| Silver(II) | |||
| Silver(III) | |||
| Silver(I,III) | |||