| |
| Names | |
|---|---|
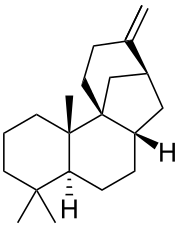
| IUPAC name (14S)-9,15:14,20-Dicyclo-8α-labd-13(16)-ene | |
| Systematic IUPAC name (4aS,6aS,8S,11aR,11bS)-4,4,11b-Trimethyl-9-methylidenetetradecahydro-8,11a-methanocycloheptanaphthalene | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| Beilstein Reference | 10812290 |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H32 |
| Molar mass | 272.476 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Stemodene is a labdane-related diterpene whose corresponding class I terpene synthase has been discovered in rice and subsequently cloned and functionally characterized. The gene responsible for stemodene production has not been found in the completed rice genome, thus suggesting that perhaps other genes are as yet undiscovered in the "completed" genome. Stemarene synthase demonstrates high sequence homology with stemodene synthase, thus accounting for the latter's discovery by Dana Morrone in 2005. Additionally, the corresponding olefin produced by each cyclase shows structural similarities and is derived from the common precursor of syn-copalyl diphosphate.
References
- Morrone, Dana; Yinghua Jin; Meimei Xu; Suh-Yeon Choi; Robert M. Coates; Reuben J. Peters (April 15, 2006). "An unexpected diterpene cyclase from rice: functional identification of a stemodene synthase" (PDF). Archives of Biochemistry and Biophysics. 448 (1–2): 133–140. doi:10.1016/j.abb.2005.09.001. PMID 16256063. Archived from the original (PDF) on 2007-09-28. Retrieved 2006-11-07.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |