
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Octanedioyl dichloride | |
| Other names Suberoyl dichloride; Suberic acid chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.156.463 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H12Cl2O2 |
| Molar mass | 211.08 g·mol |
| Density | 1.172 g/cm |
| Boiling point | 162–163 °C (324–325 °F; 435–436 K) |
| Solubility in water | Reacts with water |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H314 |
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| Flash point | 110 °C (230 °F; 383 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
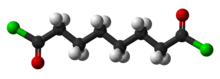
Suberoyl chloride is an organic compound with the formula (CH2)6(COCl)2. It is the diacid chloride derivative of suberic acid. It is a colorless liquid although aged samples appear yellow or even brown.
Uses
Suberoyl chloride is used as a reagent to synthesize hydroxyferrocifen hybrid compounds that have antiproliferative activity against triple negative breast cancer cells. It is also used as a cross-linking agent to cross-link chitosan membranes, and also improves the membrane's integrity.
References
- "Suberoyl chloride". Alfa Aesar. Retrieved 16 April 2019.
External links
| Diacyl chlorides (-COCl)2 | ||
|---|---|---|
|  | |
| Category:Acyl chlorides | ||
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |