| |
| Names | |
|---|---|
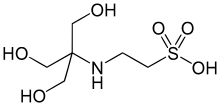
| Preferred IUPAC name 2-{amino}ethane-1-sulfonic acid | |
| Other names TES free acid | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.028.097 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H15NO6S |
| Molar mass | 229.25 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
TES is used to make buffer solutions. It has a pKa value of 7.550 (I=0, 25°C). It is one of the Good's buffers and can be used to make buffer solutions in the pH range 6.8–8.2. It is one of the components of Test yolk buffer medium used for refrigeration and transport of semen.
References
- Goldberg, R.; Kishore, N.; Lennen, R. (2002). "Thermodynamic Quantities for the Ionization Reactions of Buffers" (PDF). J. Phys. Chem. Ref. Data. 31 (2): 231–370. doi:10.1063/1.1416902.