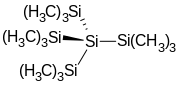
| |
| Names | |
|---|---|
| Preferred IUPAC name 1,1,1,3,3,3-Hexamethyl-2,2-bis(trimethylsilyl)trisilane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.156.064 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H36Si5 |
| Molar mass | 320.845 g·mol |
| Appearance | colorless solid |
| Density | 0.806 g/cm |
| Melting point | 319–321 °C (606–610 °F; 592–594 K) sealed tube |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tetrakis(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)4Si (where Me = CH3). It is a colorless sublimable solid with a high melting point. The molecule has tetrahedral symmetry. The compound is notable as having silicon bonded to four other silicon atoms, like in elemental silicon.
Preparation and reactions
The compound is prepared by the reaction of trimethylsilyl chloride, silicon tetrachloride, and lithium:
- 4 Me3SiCl + SiCl4 + 8 Li → (Me3Si)4Si + 8 LiCl
The compound is a precursor to tris(trimethylsilyl)silyl lithium by reaction with methyl lithium:
- (Me3Si)4Si + MeLi → (Me3Si)3SiLi + Me4Si
The organolithium compound (Me3Si)3SiLi is a versatile reagent, e.g. to tris(trimethylsilyl)silane ((Me3Si)3SiH).
See also
References
- Gilman, H.; Smith, C. L. (1967). "Tetrakis(trimethylsilyl)silane". Journal of Organometallic Chemistry. 8 (2): 245–253. doi:10.1016/S0022-328X(00)91037-4.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Joachim Dickhaut, Bernd Giese (1992). "Tris(trimethylsilyl)silane". Org. Synth. 70: 164. doi:10.15227/orgsyn.070.0164.