
| |
| |
| Names | |
|---|---|
| Other names thionitrosyl chloride | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | (NSCl)3 |
| Molar mass | 244.55 g·mol |
| Appearance | white solid |
| Melting point | 168 °C (334 °F; 441 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Trithiazyl trichloride is the inorganic compound with the formula (NSCl)3. A white solid, it is a precursor to other sulfur nitrides, but has no commercial applications.
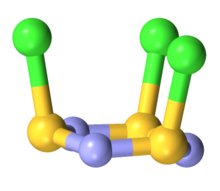
Structure
The molecule is a 6-membered ring of alternating nitrogen and sulfur atoms, where each sulfur atom is attached to one chlorine atom by a single bond. The molecule contains alternating single and double bonds in the S3N3 core. The molecule has C3v symmetry. The S3N3 core is slightly ruffled structure with S-N distances of 160.5 pm. The S-Cl distances are 208 pm, and the chlorine atoms are mutually cis. The S centers are tetravalent and pyramidal. In contrast to the NSCl connectivity, nitrosyl chloride has the connectivity ONCl.
Synthesis and reactions
Trithiazyl trichloride is obtained by chlorination of tetrasulfur tetranitride or thiazyl fluoride monomer:
- 3 S4N4 + 6 Cl2 → 4 (NSCl)3
- 3 FSN + 3 Cl2 → (NSCl)3 + 3 ClF
At 100 °C in vacuum, thiazyl chloride trimer undergoes cracking to thiazyl chloride monomer, which is a green gas.
- (−N=S(−Cl)−)3 → 3 N≡S−Cl
In N≡S−Cl, chlorine is bonded to sulfur, in contrast to nitrosyl chloride O=N–Cl, where chlorine is bonded to nitrogen. In contrast, with six fewer electrons, cyanuric chloride is a planar ring.
Alkoxide or silver salts displace the chlorides:
- (-NS(Cl)-)3 + 3 NaOR → (-NS(OR)-)3 + 3 NaCl
- (-NS(Cl)-)3 + 3 AgX → (-NS(X)-)3 + 3 AgCl
Treating thiazyl chloride with sulfur in the presence of antimony pentachloride gives dithionitronium hexachloroantimonate:
- SNCl + S + SbCl5 → [NS2]SbCl6
It reacts with nitriles to dithiadiazolium chlorides:
- 6 RCN + 4 (NSCl)3 → 6 [RCN2S2]Cl + 3 Cl2 + 3 N2
Sulfur trioxide successively oxidizes the compound at the sulfur atoms to (NSOCl)3, which exists as stereoisomers.
References
- Jolly, William L.; Maguire, Keith D. (1967). "Sulfur Nitrogen Chlorides". Inorganic Syntheses. Vol. IX. p. 102. doi:10.1002/9780470132401.ch27. ISBN 978-0-470-13240-1.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Wiegers, G. A.; Vos, A. (1966). "The Crystal Structures of Two Sulfur-Nitrogen Compounds with (S-N)3 Rings. II. Trithiazylchloride, (NSCl)3, at -130 C". Acta Crystallographica. 20 (2): 192. doi:10.1107/s0365110x66000410.
- ^ Roesky, H. W. (1971). "The Sulfur–Nitrogen Bond". In Senning, Alexander (ed.). Sulfur in Organic and Inorganic Chemistry. Vol. 1. New York: Marcel Dekker. pp. 20–23. ISBN 0-8247-1615-9. LCCN 70-154612.
- Rawson, Jeremy M.; Banister, Arthur J.; Lavender, Ian (1995). "The Chemistry of Dithiadiazolylium and Dithiadiazolyl Rings". Adv. Heterocyc. Chem. 62: 146–147. doi:10.1016/S0065-2725(08)60422-5.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.