| |
| Names | |
|---|---|
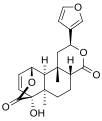
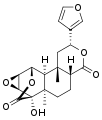
| IUPAC name (2S,4aR,6aR,7S,7aS,8aS,9S,9aS,9bS)-2-(3-Furanyl)dodecahydro-7-hydroxy-6a,9b-dimethyl-9,7-(epoxymethano)-4H-oxirenonaphthopyran-4,11-dione | |
| Other names 2,3-Epoxycolumbin; Jateorin; 5-(furan-3-yl)-12-hydroxy-3,11-dimethyl-6,14,16-trioxapentacycloheptadecane-7,17-dione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H22O7 |
| Molar mass | 374.389 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tinosporide is a chemical compound classified as a diterpenoid and a furanolactone. It was first isolated from the plant Tinospora cordifolia, from which it derives its name. It has since been found in other plants of the genus Tinospora, such as Tinospora glabra.
Because Tinospora cordifolia has been used in traditional herbal medicine, there has been research directed at exploring the potential pharmacology of tinosporide and related compounds.
Related compounds
Other diterpenoid furanolactones with a similar structure include columbin, palmarin, and chasmanthin.
External links
- Swaminathan, K.; Sinha, U. C.; Bhatt, R. K.; Sabata, B. K.; Tavale, S. S. (1989). "Structure of tinosporide, a diterpenoid furanolactone from Tinospora cordifolia Miers". Acta Crystallographica Section C. 45 (1): 134–136. Bibcode:1989AcCrC..45..134S. doi:10.1107/s0108270188009953. PMID 2610955.
- Sharma, Priyanka; Dwivedee, Bharat P.; Bisht, Dheeraj; Dash, Ashutosh K.; Kumar, Deepak (2019). "The chemical constituents and diverse pharmacological importance of Tinospora cordifolia". Heliyon. 5 (9): e02437. Bibcode:2019Heliy...502437S. doi:10.1016/j.heliyon.2019.e02437. PMC 6827274. PMID 31701036.
- "Isolation and Characterisation of clerodane diterpenoids from the traditional medicinal plant -Tinospora glabra (Burm. f.) Merrill".
- Girme, Aboli; Saste, Ganesh; Singh, Ruchi; Mirgal, Amit; Ingavale, Rajnita; Balasubramaniam, Arun Kumar; Ghoshal, Sautik; Ghule, Chetana; Patel, Saurabh; Verma, Mahendra Kumar; Maurya, Rakesh; Hingorani, Lal (2022). "Quantitative and rapid quality assessment methods for the multi-class bioactive constituents of Tinospora cordifolia using high-performance liquid and thin layer chromatography analysis with tandem mass spectrometry characterization". Separation Science Plus. 5 (8): 378–392. doi:10.1002/sscp.202200048. S2CID 249304214.
- Adib, Mohiminul; Islam, Rashedul; Ahsan, Monira; Rahman, Arifur; Hossain, Mahmud; Rahman, Md Mustafizur; Alshehri, Sultan M.; Kazi, Mohsin; Mazid, Md Abdul (2021). "Cholinesterase inhibitory activity of tinosporide and 8-hydroxytinosporide isolated from Tinospora cordifolia: In vitro and in silico studies targeting management of Alzheimer's disease". Saudi Journal of Biological Sciences. 28 (7): 3893–3900. doi:10.1016/j.sjbs.2021.03.063. PMC 8241625. PMID 34220245.
- Pathak, Ashish K.; Jain, Dharam C.; Sharma, Ram P. (1995). "Chemistry and Biological Activities of the Genera Tinospora". International Journal of Pharmacognosy. 33 (4): 277–287. doi:10.3109/13880209509065379.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |