| |
| Names | |
|---|---|
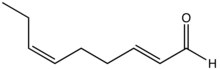
| Preferred IUPAC name (2E,6Z)-Nona-2,6-dienal | |
| Other names
(E,Z)-2,6-Nonadienal Violet leaf aldehyde Cucumber aldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.345 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H14O |
| Molar mass | 138.210 g·mol |
| Appearance | Colorless oil |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H317 |
| Precautionary statements | P261, P264, P272, P280, P302+P352, P321, P332+P313, P333+P313, P362, P363, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
trans,cis-2,6-Nonadienal is an organic compound that is classified as a doubly unsaturated derivative of nonanal. The molecule consists of a α,β-unsaturated aldehyde with an isolated alkene group. The compound has attracted attention as the essence of cucumbers, but it is also found in bread crust and freshly cut watermelon.
Biosynthesis
Isotopic labeling has indicated that nonadienal is formed from α-linolenic acid. Such reactions are typically catalyzed by hydroperoxide lyases.
See also
- 2-Nonenal - another potent odorant in cucumber
References
- Kula, Jozef; Sadowska, Halina (1993). "Unsaturated aliphatic C9-aldehydes as natural flavorants: (E,Z)-2,6-nonadienal". Perfumer & Flavorist. 18: 23–25.
- Schieberle, P.; Ofner, S.; Grosch, W. (January 1990). "Evaluation of Potent Odorants in Cucumbers (Cucumis sativus) and Muskmelons (Cucumis melo) by Aroma Extract Dilution Analysis". Journal of Food Science. 55 (1): 193–195. doi:10.1111/j.1365-2621.1990.tb06050.x.
- Cho, In Hee; Peterson, Devin G. (2010). "Chemistry of Bread Aroma: A Review". Food Science and Biotechnology. 19: 575–582. doi:10.1007/s10068-013-0240-4.
- Grosch, Werner; Schwarz, Jorg M. (May 1971). "Linoleic and linolenic acid as precursors of the cucumber flavor". Lipids. 6 (5): 351–352. doi:10.1007/BF02531828. S2CID 38868077.