| |

| |
| Names | |
|---|---|
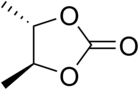
| IUPAC name trans-4,5-Dimethyl-dioxolan-2-one | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H8O3 |
| Molar mass | 116.116 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
trans-2,3-Butylene carbonate is an organic compound with formula C
5H
8O
3, or (H3C)2(C2H2)(CO3). It is an ester with a carbonate functional group bonded to both free ends of the trans-2,3-butylene group. It is also a heterocyclic compound with a five-membered ring containing two oxygen atoms, and can be viewed as a derivative of dioxolane, namely trans-4,5-dimethyl-1,3-dioxolan-2-one.
The compound is an aprotic polar solvent and has been proposed as an ingredient of the electrolyte of lithium batteries.
See also
References
- Geun-Chang Chung, Hyeong-Jin Kim, Song-Hui Jun and Myung-Hwan Kim (1999), New cyclic carbonate solvent for lithium ion batteries: trans-2,3-butylene carbonate. Electrochemistry Communications, volume 1, issue 10, pages 493-496. doi:10.1016/S1388-2481(99)00101-0