| |
| Names | |
|---|---|
| IUPAC name (2S)-2-Amino-2-acetic acid | |
| Other names α-Cycloglutamate; α-Amino-3-oxo-5-isoxazolidineacetic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H8N2O4 |
| Molar mass | 160.129 g·mol |
| Melting point | 207 °C (405 °F; 480 K) (decomp.) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
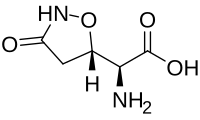
Tricholomic acid is a non-proteinogenic amino acid found in some mushrooms, including Tricholoma muscarium. It has a chemical structure similar to glutamic acid, hence the synonym cycloglutamate, and it interacts with glutamate receptors. Because glutamate receptors are thought to be responsible for the reception of umami taste, tricholomic acid and close analogs have been investigated as flavor enhancers.
See also
- Ibotenic acid, a related compound found in mushrooms
References
- ^ Takemoto, Tsunematsu; Nakajima, Tadashi (1964). "Studies on the Constituents of Indigenous Fungi. I". Yakugaku Zasshi. 84 (12): 1183–1186. doi:10.1248/yakushi1947.84.12_1183. PMID 14266548.
- Tamborini, Lucia; Mastronardi, Federica; Lo Presti, Leonardo; Nielsen, Birgitte; De Micheli, Carlo; Conti, Paola; Pinto, Andrea (2017). "Synthesis of L-Tricholomic Acid Analogues and Pharmacological Characterization at Ionotropic Glutamate Receptors". ChemistrySelect. 2 (31): 10295. doi:10.1002/slct.201702154. hdl:2434/528800.
- Kuninaka, Akira (1969). "Recent Studies of 5′-Nucleotides as New Flavor Enhancers". Flavor Chemistry. Advances in Chemistry. 56: 261–274. doi:10.1021/ba-1966-0056.ch015. ISBN 0-8412-0057-2.
External links
- Tricholomic acid, Human Metabolome Database