| |
| Names | |
|---|---|
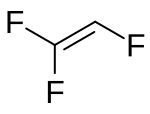
| IUPAC name Trifluoroethene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.006.025 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2HF3 |
| Molar mass | 82.025 g·mol |
| Appearance | Colourless gas |
| Density | 1.26 g/cm (liquid, at –70 °C) |
| Boiling point | −51 °C (−60 °F; 222 K) |
| Solubility | soluble in ether, slightly soluble in ethanol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H220, H280 |
| Precautionary statements | P203, P210, P222, P280, P377, P381, P403, P410+P403 |
| Related compounds | |
| Related Vinyl halides | Vinyl fluoride, vinylidene fluoride, tetrafluoroethylene, trichloroethylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Trifluoroethylene (abbreviated as TrFE) is an organofluoride compound with the chemical formula C2HF3. It is a colourless gas. TrFE can polymerise to form poly(trifluoroethylene) (PTrFE). It can also form copolymers with other monomers, such as vinylidene fluoride to form a co-polymer that is used to produce ferroelectric materials.
References
- ^ Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-500
- "Trifluoroethylene". pubchem.ncbi.nlm.nih.gov.
- Colpaert, Maxime; Banerjee, Sanjib; Ladmiral, Vincent; Ono, Taizo; Améduri, Bruno (2018). "Synthesis and properties of poly(trifluoroethylene) via a persistent radical mediated polymerization of trifluoroethylene". Polymer Chemistry. 9 (7): 894–903. doi:10.1039/c7py02018j.
- Bouad, Vincent; Guerre, Marc; Zeliouche, Sami; Améduri, Bruno; Totée, Cédric; Silly, Gilles; Poli, Rinaldo; Ladmiral, Vincent (2021). "NMR investigations of polytrifluoroethylene (PTrFE) synthesized by RAFT" (PDF). Polymer Chemistry. 12 (15): 2293–2304. doi:10.1039/d0py01753a.