| |
| Names | |
|---|---|
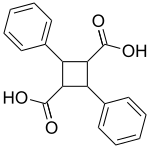
| IUPAC name 7,8′-Cyclo-8,7′-neolignane-9,9′-dioic acid | |
| Systematic IUPAC name 2,4-Diphenylcyclobutane-1,3-dicarboxylic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.022.478 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C18H16O4 |
| Molar mass | 296.322 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Truxillic acids are any of several crystalline stereoisomeric cyclic dicarboxylic acids with the formula (C6H5C2H2(CO2H)2. They are colorless solids. These compounds are obtained by the photocycloadditions of cinnamic acid where the two trans alkenes react head-to-tail. The isolated stereoisomers are called truxillic acids. The preparation of truxillic acids provided an early example of organic photochemistry.
Occurrence and reactions
These compounds are found in a variety of plants, for example in coca. Incarvillateine, an alkaloid from the plant Incarvillea sinensis, is a derivative of α-truxillic acid.
Upon heating, truxillic acids undergo cracking to give cinnamic acid.
Isomers
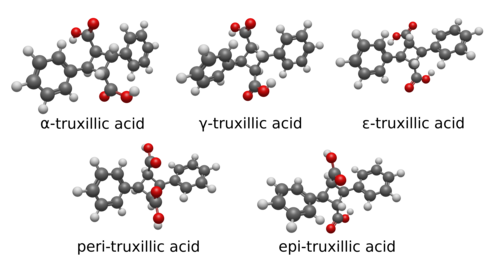
Truxillic acid can exist in five stereoisomers.

| Isomer | a | b | c | d | e | f |
|---|---|---|---|---|---|---|
| α-truxillic acid (cocaic acid) |
COOH | H | H | C6H5 | H | COOH |
| γ-truxillic acid | COOH | H | H | C6H5 | COOH | H |
| ε-truxillic acid | H | COOH | C6H5 | H | H | COOH |
| peri-truxillic acid | COOH | H | C6H5 | H | COOH | H |
| epi-truxillic acid | COOH | H | C6H5 | H | H | COOH |
Below are the five stereoisomers of truxillic acid, called alpha, gamma, epsilon, peri, and epi. These are shown both in a 2D skeletal diagram with stereocenters indicated and a 3D rendering of the structural geometry of the isomers themselves.

See also
- Truxinic acids are isomers of the truxillic acids with phenyl groups on adjacent methyne centers.
References
- Cohen, M. D.; Schmidt, G. M. J.; Sonntag, F. I. (1964). "Topochemistry. II. The photochemistry of trans-cinnamic acids". J. Chem. Soc.: 2000–2013. doi:10.1039/jr9640002000.
- Roth, Heinz D. (1989). "The Beginnings of Organic Photochemistry". Angewandte Chemie International Edition in English. 28 (9): 1193–1207. doi:10.1002/anie.198911931.
- Liebermann, C. (1888). "Ueber Cinnamylcocaïn". Berichte der Deutschen Chemischen Gesellschaft. 21 (2): 3372–3376. doi:10.1002/cber.188802102223.
- Krauze-Baranowska, Miroslawa (2002). "Truxillic and truxinic acids-occurrence in plant kingdom". Acta Poliniae Pharmaceutica-Drug Research. 59 (5): 403–410. PMID 12602803.
- Hein, Sara M. (2006). "An Exploration of a Photochemical Pericyclic Reaction Using NMR Data". Journal of Chemical Education. 83 (6): 940–942. Bibcode:2006JChEd..83..940H. doi:10.1021/ed083p940.
- Stoermer, R.; Bachér, F. (1924). "Zur Stereoisomerie der Truxillsäuren und über die Auffindung der letzten Säure dieser Gruppe (VIII.)". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 57: 15–23. doi:10.1002/cber.19240570105.
- Agarwai, O. P. (2011). Organic Chemistry Reactions and Reagents. Krishna Prakashan Media. ISBN 978-8187224655.
- "ChemSpider ID 10218892". ChemSpider. Retrieved 15 October 2016.
