| Revision as of 22:22, 28 May 2009 editThricecube (talk | contribs)Extended confirmed users30,616 editsNo edit summary← Previous edit | Revision as of 05:17, 19 June 2009 edit undoMaterialscientist (talk | contribs)Edit filter managers, Autopatrolled, Checkusers, Administrators1,994,292 edits structure and refs addedNext edit → | ||
| Line 1: | Line 1: | ||
| {{Refimprove|article|date=February 2008}} | |||
| {{Copyedit|article|date=February 2008}} | {{Copyedit|article|date=February 2008}} | ||
| {{chembox | {{chembox | ||
| | Name = Calcium carbide | | Name = Calcium carbide | ||
| | ImageFile = Carbid.jpg | | ImageFile = Carbid.jpg | ||
| | |
| ImageFile2 = BaO2structure.jpg | ||
| | ImageName = Calcium Carbide | | ImageName = Calcium Carbide | ||
| | IUPACName = Calcium Carbide | | IUPACName = Calcium Carbide | ||
| Line 22: | Line 21: | ||
| | Solubility = decomposes | | Solubility = decomposes | ||
| }} | }} | ||
| | Section3 = {{Chembox |
| Section3 = {{Chembox Structure | ||
| | CrystalStruct = Tetragonal <ref>{{cite journal| doi = 10.1023/A:1015105922260| year = 2002| author = Massalimov, I. A.| journal = Inorganic Materials| volume = 38| pages = 363}}</ref> | |||
| | SpaceGroup = D<sup>17</sup><sub>4h</sub>, I4/mmm, ] | |||
| | Coordination = 6 | |||
| }} | |||
| | Section7 = {{Chembox Hazards | |||
| | FlashPoint = 305 °C (acetylene) | | FlashPoint = 305 °C (acetylene) | ||
| | EUClass = | | EUClass = | ||
| Line 34: | Line 38: | ||
| }} | }} | ||
| '''Calcium carbide''' is a ] with the ] of ]]. The material is colorless, but most samples appear black through to grayish white lumps, depending on the grade. Its main use industrially is in the production of ] and ]<ref> |
'''Calcium carbide''' is a ] with the ] of ]]. The material is colorless, but most samples appear black through to grayish white lumps, depending on the grade. Its main use industrially is in the production of ] and ]<ref name=handbook>{{cite book | last =Patnaik | first =Pradyot | year = 2003 | title =Handbook of Inorganic Chemical Compounds | publisher = McGraw-Hill | pages = | isbn =0070494398 | url= http://books.google.com/books?id=Xqj-TTzkvTEC&pg=PA243 | accessdate = 2009-06-06}}</ref> | ||
| ==Production== | ==Production== | ||
| Line 86: | Line 90: | ||
| ===Carbide lamps=== | ===Carbide lamps=== | ||
| {{Main|Carbide lamp}}] | {{Main|Carbide lamp}}] | ||
| Calcium carbide is used in ]s, in which water drips on carbide and the formed acetylene is ignited. These lamps were unusable in coal mines where the presence of the flammable gas methane made them a serious hazard. The presence of flammable gases in coal mines led to the miner ]. However carbide lamps were used extensively in slate, copper and tin mines, but most have now been replaced by electric lamps. Carbide lamps are still used by some ]s ] and other underground areas,<ref> |
Calcium carbide is used in ]s, in which water drips on carbide and the formed acetylene is ignited. These lamps were unusable in coal mines where the presence of the flammable gas methane made them a serious hazard. The presence of flammable gases in coal mines led to the miner ]. However carbide lamps were used extensively in slate, copper and tin mines, but most have now been replaced by electric lamps. Carbide lamps are still used by some ]s ] and other underground areas,<ref>{{cite web| url = http://www.teara.govt.nz/TheBush/BushAndMountainRecreation/Caving/4/en | title =Caving equipment and culture (from ])}}</ref> though they are increasingly being replaced in this use by ] lights. They were also used extensively as head lights in early ], although in this application they are also obsolete, having been replaced entirely by electric lamps.<ref>{{cite book| author = Clemmer, Gregg| title = American Miners' Carbide Lamps: A Collectors Guide to American Carbide Mine Lighting| publisher = Westernlore Publications| year = 1987}}</ref> | ||
| ===Other uses=== | ===Other uses=== | ||
| In the ripening of fruit, it is used as source of acetylene gas, which is a ripening agent (similar to ]).<ref>{{cite journal | author = F. B. Abeles and H. E. Gahagan, III | title = Abscission: The Role of Ethylene, Ethylene Analogues, Carbon Dioxide, and Oxygen | year = 1968 | journal = ] | volume = 43 | issue = 8 | pages = 1255–1258 | url = http://www.plantphysiol.org/cgi/content/abstract/43/8/1255 | pmid = 16656908 | doi = 10.1104/pp.43.8.1255 }}</ref> | In the ripening of fruit, it is used as source of acetylene gas, which is a ripening agent (similar to ]).<ref>{{cite journal | author = F. B. Abeles and H. E. Gahagan, III | title = Abscission: The Role of Ethylene, Ethylene Analogues, Carbon Dioxide, and Oxygen | year = 1968 | journal = ] | volume = 43 | issue = 8 | pages = 1255–1258 | url = http://www.plantphysiol.org/cgi/content/abstract/43/8/1255 | pmid = 16656908 | doi = 10.1104/pp.43.8.1255 }}</ref> | ||
| It is still used in the ] and ] for a traditional custom called ''Carbidschieten'' (Shooting Carbide). To create an explosion, carbide and water are put in a milk churn with a lid. Ignition is usually done with a torch. Some villages in the Netherlands fire multiple milk churns in a row as a New Year's Eve tradition.{{ |
It is still used in the ] and ] for a traditional custom called ''Carbidschieten'' (Shooting Carbide). To create an explosion, carbide and water are put in a milk churn with a lid. Ignition is usually done with a torch. Some villages in the Netherlands fire multiple milk churns in a row as a New Year's Eve tradition.<ref>{{cite web|url = http://www.carbidbus.nl/Carbid1/Carbidschieten_The_dutch_favou/carbidschieten_the_dutch_favou.html| title =an explanation of Carbid Schieten|accessdate = 2009-06-06}}</ref> The old tradition comes from the old ] religion to chase off spirits. | ||
| It is used in toy cannons (see ]), as well as in ]s. | It is used in toy cannons (see ]), as well as in ]s. | ||
| Line 104: | Line 108: | ||
| ==References== | ==References== | ||
| {{reflist}} | |||
| <references /> | |||
| {{Calcium compounds}} | {{Calcium compounds}} | ||
Revision as of 05:17, 19 June 2009
| This article may require copy editing for grammar, style, cohesion, tone, or spelling. You can assist by editing it. (February 2008) (Learn how and when to remove this message) |

| |
| |
| Names | |
|---|---|
| IUPAC name Calcium Carbide | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.000.772 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | CaC2 |
| Molar mass | 64.099 g/mol |
| Appearance | White powder to grey/black crystals |
| Density | 2.22 g/cm |
| Melting point | 2,160 °C (3,920 °F; 2,430 K) |
| Boiling point | 2,300 °C (4,170 °F; 2,570 K) |
| Solubility in water | decomposes |
| Structure | |
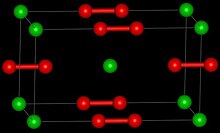
| Crystal structure | Tetragonal |
| Space group | D4h, I4/mmm, tI6 |
| Coordination geometry | 6 |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Calcium carbide is a chemical compound with the chemical formula of CaC2. The material is colorless, but most samples appear black through to grayish white lumps, depending on the grade. Its main use industrially is in the production of acetylene and calcium cyanamide
Production
Calcium carbide is produced industrially in an electric arc furnace loaded with a mixture of lime and coke at approximately 2000 °C. This method has not changed since its invention in 1888:
- CaO + 3C → CaC2 + CO
The high temperature required for this reaction is not practically achievable by traditional combustion, so the reaction is performed in an electric arc furnace with graphite electrodes. The carbide product produced generally contains around 80% calcium carbide by weight. The carbide is crushed to produce small lumps that can range a few mm up to 50 mm. The impurities are concentrated in the finer fractions. The CaC2 content of the product is assayed by measuring the amount of acetylene produced on hydrolysis. As an example the British and German standards for the content of the coarser fractions are 295 L/kg and 300 L/kg respectively. Impurities present in the carbide include phosphide, which produces phosphine when hydrolysed.
This reaction was an important part of the industrial revolution in chemistry. In the USA this occurred as a product of massive amounts of cheap hydro-electric power liberated from Niagara Falls before the turn of the 20th century.
The method for the production in an electric arc furnace was discovered independently by T. L Willson and H. Moissan in 1888 and 1892.
Crystal structure
Pure calcium carbide is a colourless solid. The common crystalline form at room temperature is a distorted rock salt structure with the C2 units lying parallel.
Applications
Production of acetylene
The reaction of calcium carbide with water was discovered by Friedrich Wöhler in 1862.
- CaC2 + 2H2O → C2H2 + Ca(OH)2
This reaction is the basis of the industrial manufacture of acetylene, and is the major industrial use of calcium carbide. In China, acetylene derived from calcium carbide remains a feedstock for the chemical industry, in particular for the production of polyvinyl chloride, PVC. Locally produced acetylene is more economic than using imported oil. Production of calcium carbide in China has been increasing. In 2005 output was 8.94 million tons with capacity to produce 17 million tons. In the USA, Europe and Japan consumption is generally declining. Production levels in the USA in 1990 were 236,000 tons pa.
Production of calcium cyanamide
Calcium carbide reacts with nitrogen at high temperature to form calcium cyanamide:
- CaC2 + N2 → CaCN2 + C
Calcium cyanamide is used as fertilizer. It is hydrolysed to cyanamide, H2NCN.
Steelmaking
Calcium carbide is used:
- in the desulfurisation of iron (pig iron, cast iron and steel)
- as a fuel in steelmaking to extend the scrap ratio to liquid iron depending on economics.
- as a powerful deoxidizer at ladle treatment facilities.
Carbide lamps
Main article: Carbide lamp
Calcium carbide is used in carbide lamps, in which water drips on carbide and the formed acetylene is ignited. These lamps were unusable in coal mines where the presence of the flammable gas methane made them a serious hazard. The presence of flammable gases in coal mines led to the miner safety lamp. However carbide lamps were used extensively in slate, copper and tin mines, but most have now been replaced by electric lamps. Carbide lamps are still used by some cavers exploring caves and other underground areas, though they are increasingly being replaced in this use by LED lights. They were also used extensively as head lights in early automobiles, although in this application they are also obsolete, having been replaced entirely by electric lamps.
Other uses
In the ripening of fruit, it is used as source of acetylene gas, which is a ripening agent (similar to ethylene).
It is still used in the Netherlands and Belgium for a traditional custom called Carbidschieten (Shooting Carbide). To create an explosion, carbide and water are put in a milk churn with a lid. Ignition is usually done with a torch. Some villages in the Netherlands fire multiple milk churns in a row as a New Year's Eve tradition. The old tradition comes from the old pagan religion to chase off spirits.
It is used in toy cannons (see Big-Bang Cannon), as well as in bamboo cannons.
Together with calcium phosphide, calcium carbide is used in floating, self-igniting naval signal flares (see Holmes' Marine Life Protection Association).
Calcium carbide is also used in small carbide lamps called carbide candles, which are used for blackening rifle sights to reduce glare. These "candles" are used due to the sooty flame produced by acetylene.
External links
References
- Massalimov, I. A. (2002). Inorganic Materials. 38: 363. doi:10.1023/A:1015105922260.
{{cite journal}}: Missing or empty|title=(help) - Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. ISBN 0070494398. Retrieved 2009-06-06.
- ^ Calcium Carbide, Bernhard Langhammer, Ullmann's Encyclopedia of Industrial Chemistry, Wiley Interscience. (Subscription required)
- J. T. Morehead, G. de Chalmot (1896). "The Manufacture of Calcium carbide". Journal of the American Chemical Society. 18 (4): 311–331. doi:10.1021/ja02090a001.
- H. Moissan (1892). "Chimie Minérale.- Description d'un nouveau four électrique". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 115: 1031.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Ya Dun (2006-01-23). "Troubles in the PVC industry". Hong Kong Trade Development Council.
- "Govt takes measures to curb development of calcium carbide sector". BusyTrade.com. 2007-05-16.
- Jamie Lacson, Stefan Schlag and Goro Toki (2004). "Calcium Carbide". SRI Consulting.
{{cite web}}: Unknown parameter|month=ignored (help) - "Caving equipment and culture (from [[Te Ara Encyclopedia of New Zealand]])".
{{cite web}}: URL–wikilink conflict (help) - Clemmer, Gregg (1987). American Miners' Carbide Lamps: A Collectors Guide to American Carbide Mine Lighting. Westernlore Publications.
- F. B. Abeles and H. E. Gahagan, III (1968). "Abscission: The Role of Ethylene, Ethylene Analogues, Carbon Dioxide, and Oxygen". Plant Physiol. 43 (8): 1255–1258. doi:10.1104/pp.43.8.1255. PMID 16656908.
- "an explanation of Carbid Schieten". Retrieved 2009-06-06.
| Calcium compounds | |
|---|---|
| Hydrogen & halogens | |
| Chalcogens | |
| Pnictogens | |
| Group 13 & 14 | |
| Trans metals | |
| Organics | |