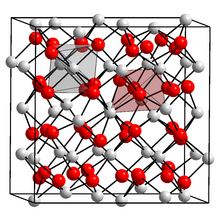
 Unit cell containing 31 nitride ions (red) and 48 calcium ions (white). Each nitride is surrounded by six calcium, and each calcium by four nitride ions. | |
| Names | |
|---|---|
| IUPAC name Calcium nitride | |
| Other names tricalcium dinitride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.435 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Ca3N2 |
| Molar mass | 148.248 g·mol |
| Appearance | red-brown crystalline solid |
| Density | 2.670 g/cm 2.63 g/cm (17 °C) |
| Melting point | 1,195 °C (2,183 °F; 1,468 K) |
| Solubility in water | decomposes |
| Structure | |
| Crystal structure | Cubic, cI80 |
| Space group | Ia-3, No. 206 |
| Related compounds | |
| Other anions | |
| Other cations | Beryllium nitride Magnesium nitride Strontium nitride Barium nitride Radium nitride Zinc nitride Aluminium nitride Lithium nitride Sodium nitride Potassium nitride |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Calcium nitride is the inorganic compound with the chemical formula Ca3N2. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered.
Structure
α-Calcium nitride adopts an anti-bixbyite structure, similar to Mn2O3, except that the positions of the ions are reversed: calcium (Ca) take the oxide (O) positions and nitride ions (N) the manganese (Mn). In this structure, Ca occupies tetrahedral sites, and the nitride centres occupy two different types of octahedral sites.
Synthesis and reactions
Calcium nitride is formed along with the oxide, CaO, when calcium burns in air. It can be produced by direct reaction of the elements:
- 3 Ca + N2 → Ca3N2
It reacts with water or even the moisture in air to give ammonia and calcium hydroxide:
- Ca3N2 + 6 H2O → 3 Ca(OH)2 + 2 NH3
Like sodium oxide, calcium nitride absorbs hydrogen above 350 °C:
- Ca3N2 + 2 H2 → 2 CaNH + CaH2
General references
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
References
- Eagleson, M. (1994). Concise Encyclopedia Chemistry. Walter de Gruyter. p. 160. ISBN 3-11-011451-8.
Calcium nitride.
- Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- P. Ehrlich “Calcium, Strontium, Barium Nitrides Ca3N2, Sr3N2, Ba3N2” in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 940-1.
- Heyns, A. (1998). "The Vibrational Spectra and Decomposition of α-Calcium Nitride (α-Ca3N2) and Magnesium Nitride (Mg3N2)". Journal of Solid State Chemistry. 137 (1): 33–41. Bibcode:1998JSSCh.137...33H. doi:10.1006/jssc.1997.7672.
External links
| Calcium compounds | |
|---|---|
| Hydrogen & halogens | |
| Chalcogens | |
| Pnictogens | |
| Group 13 & 14 | |
| Trans metals | |
| Organics | |
| Salts and covalent derivatives of the nitride ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |