| |
| Names | |
|---|---|
| IUPAC name Zirconium nitride | |
| Other names Zirconium(III) nitride, Nitridozirconium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.042.864 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ZrN |
| Appearance | Yellow-brown crystals |
| Odor | Odorless |
| Density | 7.09 g/cm (24 °C) |
| Melting point | 2,952 °C (5,346 °F; 3,225 K) at 760 mmHg |
| Solubility in water | Insoluble |
| Solubility | Soluble in concentrated HF, acids |
| Structure | |
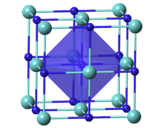
| Crystal structure | Cubic, cF8 |
| Space group | Fm3m, No. 225 |
| Lattice constant | a = 4.5675 Åα = 90°, β = 90°, γ = 90° |
| Coordination geometry | Octahedral |
| Thermochemistry | |
| Heat capacity (C) | 40.442 J/mol·K |
| Std molar entropy (S298) |
38.83 J/mol·K |
| Std enthalpy of formation (ΔfH298) |
−365.26 kJ/mol |
| Related compounds | |
| Related refractory ceramic materials | Tantalum carbide Niobium carbide Zirconium carbide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Zirconium nitride (ZrN) is an inorganic compound used in a variety of ways due to its properties.
Properties
ZrN grown by physical vapor deposition (PVD) is a light gold color similar to elemental gold. ZrN has a room-temperature electrical resistivity of 12.0 μΩ·cm, a temperature coefficient of resistivity of 5.6·10 Ω·cm/K, a superconducting transition temperature of 10.4 K, and a relaxed lattice parameter of 0.4575 nm. The hardness of single-crystal ZrN is 22.7±1.7 GPa and elastic modulus is 450 GPa.
Uses

Zirconium nitride is a hard ceramic material similar to titanium nitride and is a cement-like refractory material. Thus it is used in cermets and laboratory crucibles. When applied using the physical vapor deposition coating process it is commonly used for coating medical devices, industrial parts (notably drill bits), automotive and aerospace components and other parts subject to high wear and corrosive environments.
Zirconium nitride was suggested as a hydrogen peroxide fuel tank liner for rockets and aircraft.
References
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Sirajuddeen, M. Md. Sheik.; Banu, I. B. S. (2014). "FP-LAPW investigation of electronic, magnetic, elastic and thermal properties of Fe-doped zirconium nitride". AIP Advances. 4 (5): 057121. Bibcode:2014AIPA....4e7121S. doi:10.1063/1.4879798.
- ^ Zirconium nitride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-06-30)
- Mei, A. B.; Howe, B. M.; Zhang, C.; Sardela, M.; Eckstein, J. N.; Hultman, L.; Rockett, A.; Petrov, I.; Greene, J. E. (2013). "Physical properties of epitaxial ZrN/MgO(001) layers grown by reactive magnetron sputtering". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 31 (6): 061516. Bibcode:2013JVSTA..31f1516M. doi:10.1116/1.4825349.
- Slate, A. J.; Wickens, D. J.; El Mohtadi, M.; Dempsey-Hibbert, N.; West, G.; Banks, C. E.; Whitehead, K. A. (2018). "Slate, A.J., Wickens, D.J., El Mohtadi, M. et al. Antimicrobial activity of Ti-ZrN/Ag coatings for use in biomaterial applications. Sci Rep 8, 1497 (2018)". Scientific Reports. 8 (1): 1497. doi:10.1038/s41598-018-20013-z. PMC 5784091. PMID 29367635.
- US 7736751, Yousefiani, Ali, "Coating for components requiring hydrogen peroxide compatibility", published 2010-06-15, assigned to Boeing Co.
| Zirconium compounds | |||||
|---|---|---|---|---|---|
| Zr(II) | |||||
| Zr(III) | |||||
| Zr(IV) |
| ||||
| Salts and covalent derivatives of the nitride ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||