| |
| Names | |
|---|---|
| Other names tetrakis(acetylacetonato)zirconium, zirconium tetraacetylacetonate, zirconium tetrakis(acetylacetonate), tetrakis(acetylacetonato) zirconium, Zirconium(IV) 2,4-pentanedionate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.037.721 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H28O8Zr |
| Molar mass | 487.660 g·mol |
| Appearance | white solid |
| Density | 1.419 g/cm |
| Melting point | 194–195 °C (381–383 °F; 467–468 K) |
| Sublimation conditions |
140 °C in vacuo |
| Solubility in benzene | 200 g/L |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H315, H319, H332, H335 |
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
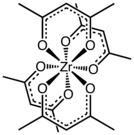
Zirconium acetylacetonate is the coordination complex with the formula Zr(C5H7O2)4. It is a common acetylacetonate of zirconium. It is a white solid that exhibits high solubility in nonpolar organic solvents, but not simple hydrocarbons.
The complex is prepared by treating zirconium oxychloride with acetylacetone:
- ZrOCl2 + 4 Hacac → Zr(acac)4 + 2 HCl + H2O
The complex has a square antiprismatic geometry with eight nearly equivalent Zr-O bonds of length 2.19 Å. The molecular symmetry is D2, i.e. the complex is chiral. Compounds of high coordination number tend to be stereochemically nonrigid as indicated by the observation of one methyl signal by proton NMR spectroscopy.
More volatile than Zr(acac)4 is the related complex of 1,1,1-trifluoroacetylacetonate.
References
- ^ Young, R. C.; Arch, Arnold (1946). "Zirconium Acetylacetonate [Tetrakis(2,4-pentanediono)zirconium]". Inorganic Syntheses. Vol. 2. pp. 121–148. doi:10.1002/9780470132333.ch35. ISBN 978-0-470-13233-3.
{{cite book}}:|journal=ignored (help) - Clegg, William (1987). "Redetermination of the structure of tetrakis(acetylacetonato)zirconium(IV)". Acta Crystallographica Section C. 43 (4): 789–91. doi:10.1107/S0108270187094083.
- ^ Morris, Melvin L.; Moshier, Ross W.; Sievers, Robert E. (1967). "Tetrakis(1,1,1-trifluoro-2,4-pentanedionato)zirconium(and Hafnium)". Tetrakis(1,1,1-trifluoro-2,4-pentanedionato)zirconium (and Hafnium). Inorganic Syntheses. Vol. 9. pp. 50–52. doi:10.1002/9780470132401.ch15. ISBN 978-0-470-13168-8.
| Zirconium compounds | |||||
|---|---|---|---|---|---|
| Zr(II) | |||||
| Zr(III) | |||||
| Zr(IV) |
| ||||