| Revision as of 14:07, 6 August 2010 view sourceBrews ohare (talk | contribs)47,831 edits Avoid suggesting mass and volume is an adequate definition; keep the basic notion general to include later ones← Previous edit | Revision as of 14:21, 6 August 2010 view source Brews ohare (talk | contribs)47,831 editsm →Dark energy: fix Smolin url to link to page 16Next edit → | ||
| Line 1,142: | Line 1,142: | ||
| |year=2007 | |year=2007 | ||
| |title=The Trouble with Physics | |title=The Trouble with Physics | ||
| |url=http://books.google.com/?id=z5rxrnlcp3sC&pg= |
|url=http://books.google.com/?id=z5rxrnlcp3sC&pg=PA16 | ||
| |page=16 | |page=16 | ||
| |publisher=] | |publisher=] | ||
Revision as of 14:21, 6 August 2010
- This article is about the concept in physical sciences. For other uses, see Matter (disambiguation).
Matter is that which comprises physical objects – the “things” of the world. Typically, atoms, molecules and other particles are included. However, there is no single agreed upon scientific meaning; each field uses the term in different and sometimes conflicting ways. In common usage, matter often is defined as that which has mass and occupies volume; However, this popular definition is only one of many possibilities discussed in this article, and is incomplete from a scientific viewpoint, as described in detail in the two sections below on historical development and definitions.
For much of the history of the natural sciences people have contemplated the exact nature of matter. The idea that matter was build from discrete building blocks, the so-called particulate theory of matter, was first put forward by the Greek philosophers Leucippus (~490 BC) and Democritus (~470–380 BC). Over time an increasingly fine structure for matter was discovered: objects are made from molecules, molecules consist of atoms, which in turn consist of subatomic particles like protons and electrons.
Matter is commonly said to exist in four states (or phases): solid, liquid, gas and plasma. However, advances in experimental technique have realized other phases, previously only theoretical constructs, such as Bose–Einstein condensates and Fermionic condensates. A focus on an elementary-particle view of matter also leads to new phases of matter, such as the quark–gluon plasma.
In physics and chemistry, matter exhibits both wave-like and particle-like properties, the so-called wave–particle duality.
In the realm of cosmology, extensions of the term matter are invoked to include dark matter and dark energy, concepts introduced to explain some odd phenomena of the observable universe, such as the galactic rotation curve. These exotic forms of "matter" do not refer to matter as "building blocks", but rather to currently poorly understood forms of mass and energy.
Historical development
Origins
The pre-Socratics were among the first recorded speculators about the underlying nature of the visible world. Thales (c. 624 BC–c. 546 BC) regarded water as the fundamental material of the world. Anaximander (c. 610 BC–c. 546 BC) posited that the basic material was wholly characterless or limitless: the Infinite (apeiron). Anaximenes (flourished 585 BC, d. 528 BC) posited that the basic stuff was pneuma or air. Heraclitus (c. 535–c. 475 BC) seems to say the basic element is fire, though perhaps he means that all is change. Empedocles (c. 490–430 BC) spoke of four basic materials of which everything was made: earth, water, air, and fire. Meanwhile, Parmenides argued that change does not exist, and Democritus argued that everything is composed of minuscule, inert bodies of all shapes called atoms. All of these notion had deep philosophical problems.
Aristotle (384 BC – 322 BC) was the first to put the conception on a sound philosophical basis, which he did in his natural philosophy, especially in Physics book I. He adopted as reasonable suppositions the four Empedoclean elements, but added a fifth, aether. Nevertheless these elements are not basic in Aristotle's mind. Rather they, like everything else in the visible world, are composed of the basic principles matter and form.
The word Aristotle uses for matter, ὑλη (hyle or hule), can be literally translated as wood or timber, that is, "raw material" for building. Indeed, Aristotle's conception of matter is intrinsically linked to something being made or composed. In other words, in contrast to the early modern conception of matter as simply occupying space, matter for Aristotle is definitionally linked to process or change: matter is what underlies a change of substance.
For example, a horse eats grass: the horse changes the grass into itself; the grass as such does not persist in the horse, but some aspect of it—its matter—does. The matter is not specifically described (e.g., as atoms), but consists of whatever persists in the change of substance from grass to horse. Matter in this understanding does not exist independently (i.e., as a substance), but exists interdependently (i.e., as a "principle") with form and only insofar as it underlies change. It can be helpful to conceive of the relationship of matter and form as very similar to that between parts and whole. For Aristotle, matter as such can only receive actuality from form; it has no activity or actuality in itself, similar to the way that parts as such only exist in a whole (otherwise they would be independent wholes).
Early modernity
René Descartes (1596–1650) was the originator of the modern conception of matter. Being a geometer, he redefined matter to be suitable for abstract, mathematical treatment as that which occupies space:
So, extension in length, breadth, and depth, constitutes the nature of bodily substance; and thought constitutes the nature of thinking substance. And everything else which can be attributed to body presupposes extension, and is only a mode of that which is extended
— René Descartes, Principles of Philosophy
For Descartes, matter has only the property of extension, so its only activity aside from locomotion is to exclude other bodies: this is the mechanical philosophy. Descartes makes an absolute distinction between mind, which he defines as unextended, thinking substance, and matter, which he defines as unthinking, extended substance. They are independent things. In contrast, Aristotle defines matter and the formal/forming principle as complementary principles which together compose one independent thing (substance). In short, Aristotle defines matter (roughly speaking) as what things are made of, but Descartes elevates matter to be a thing in itself.
The continuity and difference between Descartes' and Aristotle's conceptions is noteworthy. In both conceptions, matter is passive or inert. In the respective conceptions matter has different relationships to intelligence. For Aristotle, matter and intelligence (form) exist together in an interdependent relationship, whereas for Descartes, matter and intelligence (mind) are definitionally opposed, independent substances.
Isaac Newton (1643–1727) inherited Descartes' mechanical conception of matter; he viewed matter as "solid, massy, hard, impenetrable, movable particles", which were "even so very hard as never to wear or break in pieces." The "primary" properties of matter were amenable to mathematical description, unlike "secondary" qualities such as color or taste. Newton restores to matter intrinsic properties in addition to extension (at least on a limited basis), such as mass. A key distinction between Descartes's and Newton's views was that Newton refuted Descartes' contact mechanics by showing that bodies have capacities like attraction (notably, gravity). Along the same lines, Joseph Priestly argued that corporeal properties transcend contact mechanics: chemical properties require the capacity for attraction.. In the 19th century, following the development of the periodic table, and of atomic theory, atoms were seen as being the fundamental constituents of matter; atoms formed molecules and compounds.
Chomsky summarizes the situation:
What is the concept of body that finally emerged? The answer is that there is no clear and definite conception of body. Rather, the material world is whatever we discover it to be, with whatever properties it must be assumed to have for the purposes of explanatory theory. Any intelligible theory that offers genuine explanations and that can be assimilated to the core notions of physics becomes part of the theory of the material world, part of our account of body. If we have such a theory in some domain, we seek to assimilate it to the core notions of physics, perhaps modifying these notions as we carry out this enterprise.
— Noam Chomsky, Language and problems of knowledge: the Managua lectures, p. 144
Late nineteenth and early twentieth century
The common definition in terms of occupying space and having mass is in contrast with most physical and chemical definitions of matter, which rely instead upon its structure and upon attributes not necessarily related to volume and mass. At the turn of the nineteenth century, the concept of matter began a rapid evolution.
Aspects of the Newtonian view still held sway. James Clerk Maxwell discussed matter in his work Matter and Motion. He carefully separates "matter" from space and time, and defines it in terms of the object referred to in Newton's first law of motion.
However, the Newtonian picture was not the whole story. In the 19th century, the term "matter" was actively discussed by a host of scientists and philosophers, and a brief outline can be found in Levere. A textbook discussion from 1870 suggests matter is what is made up of atoms:
Three divisions of matter are recognized in science: masses, molecules and atoms.
A Mass of matter is any portion of matter appreciable by the senses.
A Molecule is the smallest particle of matter into which a body can be divided without losing its identity.
An Atom is a still smaller particle produced by division of a molecule.
Rather than simply having the attributes of mass and occupying space, matter was held to have chemical and electrical properties. The famous physicist J. J. Thomson wrote about the "constitution of matter" and was concerned with the possible connection between matter and electrical charge. There is an entire literature concerning the "structure of matter", ranging from the "electrical structure" in the early 20th century, to the more recent "quark structure of matter", introduced today with the remark: Understanding the quark structure of matter has been one of the most important advances in contemporary physics. In this connection, physicists speak of matter fields, and speak of particles as "quantum excitations of a mode of the matter field". And here is a quote from de Sabbata and Gasperini: "With the word "matter" we denote, in this context, the sources of the interactions, that is spinor fields (like quarks and leptons), which are believed to be the fundamental components of matter, or scalar fields, like the Higgs particles, which are used to introduced mass in a gauge theory (and which, however, could be composed of more fundamental fermion fields)."
Later developments
The modern conception of matter has been refined many times in history, in light of the improvement in knowledge of just what the basic building blocks are, and in how they interact.
In the late 19th century with the discovery of the electron, and in the early 20th century, with the discovery of the atomic nucleus, and the birth of particle physics, matter was seen as made up of electrons, protons and neutrons interacting to form atoms. Today, we know that even protons and neutrons are not indivisible, they can be divided into quarks, while electrons are part of a particle family called leptons. Both quarks and leptons are elementary particles, and are currently seen as being the fundamental constituents of matter.
These quarks and leptons interact through four fundamental forces: gravity, electromagnetism, weak interactions, and strong interactions. The Standard Model of particle physics is currently the best explanation for all of physics, but despite decades of efforts, gravity cannot yet be accounted for at the quantum-level; it is only described by classical physics (see quantum gravity and graviton). Interactions between quarks and leptons are the result of an exchange of force-carrying particles (such as photons) between quarks and leptons. The force-carrying particles are not themselves building blocks. As one consequence, mass and energy (which cannot be created or destroyed) cannot always be related to matter (which can be created out of non-matter particles such as photons, or even out of pure energy, such as kinetic energy). Force carriers are usually not considered matter: the carriers of the electric force (photons) possess energy (see Planck relation) and the carriers of the weak force (W and Z bosons) are massive, but neither are considered matter either. However, while these particles are not considered matter, they do contribute to the total mass of atoms, subatomic particles, and all systems which contain them.
Summary
The term "matter" is used throughout physics in a bewildering variety of contexts: for example, one refers to "condensed matter physics", "elementary matter", "partonic" matter, "dark" matter, "anti"-matter, "strange" matter, and "nuclear" matter. In discussions of matter and antimatter, normal matter has been referred to by Alfvén as koinomatter. It is fair to say that in physics, there is no broad consensus as to an exact definition of matter, and the term "matter" usually is used in conjunction with some modifier.
Definitions
Common definition
The common definition of matter is anything that has both mass and volume (occupies space). For example, a car would be said to be made of matter, as it occupies space, and has mass.
The observation that matter occupies space goes back to antiquity. However, an explanation for why matter occupies space is recent, and is argued to be a result of the Pauli exclusion principle. Two particular examples where the exclusion principle clearly relates matter to the occupation of space are white dwarf stars and neutron stars, discussed further below.
Amount of substance
The international standards organization Bureau International des Poids et Mesures (BIPM) uses the terminology "amount of substance", rather than "matter". To quote the SI brochure:
"Amount of substance is defined to be proportional to the number of specified elementary entities in a sample, the proportionality constant being a universal constant which is the same for all samples. The unit of amount of substance is called the mole, symbol mol, and the mole is defined by specifying the mass of carbon 12 that constitutes one mole of carbon 12 atoms. By international agreement this was fixed at 0.012 kg, i.e. 12 g.
- 1. The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is "mol".
- 2. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles."
Atoms and molecules definition
A definition of "matter" that is based upon its physical and chemical structure is: matter is made up of atoms and molecules. This definition is consistent with the BIPM definition of "amount of substance" above, but is more specific about the constituents of matter. (For further discussion, see the sections Late nineteenth and early twentieth century and Quarks and leptons definition). As an example, deoxyribonucleic acid molecules (DNA) are matter under this definition because they are made of atoms. This definition can be extended to include charged atoms and molecules, so as to include plasmas (gases of ions) and electrolytes (ionic solutions), which are not obviously included in the atoms and molecules definition. Alternatively, one can adopt the protons, neutrons and electrons definition.
Protons, neutrons and electrons definition
A definition of "matter" more fine-scale than the atoms and molecules definition is: matter is made up of what atoms and molecules are made of, meaning anything made of protons, neutrons, and electrons. This definition goes beyond atoms and molecules, however, to include substances made from these building blocks that are not simply atoms or molecules, for example white dwarf matter — typically, carbon and oxygen nuclei in a sea of degenerate electrons. At a microscopic level, the constituent "particles" of matter such as protons, neutrons and electrons obey the laws of quantum mechanics and exhibit wave–particle duality. At an even deeper level, protons and neutrons are made up of quarks and the force fields (gluons) that bind them together (see Quarks and leptons definition below).
Quarks and leptons definition

As may be seen from the above discussion, many early definitions of what can be called ordinary matter were based upon its structure or "building blocks". On the scale of elementary particles, a definition that follows this tradition can be stated as: ordinary matter is everything that is composed of elementary fermions, namely quarks and leptons. The connection between these formulations follows.
Leptons (the most famous being the electron), and quarks (of which baryons, such as protons and neutrons, are made) combine to form atoms, which in turn form molecules. Because atoms and molecules are said to be matter, it is natural to phrase the definition as: ordinary matter is anything that is made of the same things that atoms and molecules are made of. (However, notice that one also can make from these building blocks matter that is not atoms or molecules.) Then, because electrons are leptons, and protons and neutrons are made of quarks, this definition in turn leads to the definition of matter as being "quarks and leptons", which are the two types of elementary fermions. Carithers and Grannis state: Ordinary matter is composed entirely of first-generation particles, namely the and quarks, plus the electron and its neutrino. (Higher generations particles quickly decay into first-generation particles, and thus are not commonly encountered.)
This definition of ordinary matter is more subtle than it first appears. All the particles that make up ordinary matter (leptons and quarks) are elementary fermions, while all the force carriers are elementary bosons.. The W and Z bosons that mediate the weak force are not made of quarks or leptons, and so are not ordinary matter, even if they have mass. In other words, mass is not something that is exclusive to ordinary matter.
The quark–lepton definition of ordinary matter, however, identifies not only the elementary building blocks of matter, but also includes composites made from the constituents (atoms and molecules, for example). Such composites contain an interaction energy that holds the constituents together, and may constitute the bulk of the mass of the composite. As an example, to a great extent, the mass of an atom is simply the sum of the masses of its constituent protons, neutrons and electrons. However, digging deeper, the protons and neutrons are made up of quarks bound together by gluon fields (see dynamics of quantum chromodynamics) and these gluons fields contribute significantly to the mass of hadrons. In other words most of what composes the "mass" of ordinary matter is due to the binding energy of quarks within protons and neutrons. For example, the sum of the mass of the three quarks in a nucleon is approximately 12.5 MeV/c, which is low compared to the mass of a nucleon (approximately 938 MeV/c). The bottom line is that most of the mass of everyday objects comes from the interaction energy of its elementary components.
Smaller building blocks?
“In the past, the search for building blocks of matter has led us to more and more 'elementary' entities – from the molecule to the atom, to the nucleus and electrons, to the nucleons, and eventually to the quarks. Have we completed this 'onion peeling' process ... ?” The Standard Model groups matter particles into three generations, where each generation consists of two quarks and two leptons. The first generation is the up and down quarks, the electron and the electron neutrino; the second includes the charm and strange quarks, the muon and the muon neutrino; the third generation consists of the top and bottom quarks and the tau and tau neutrino. “... the most natural explanation to the existence of higher generations of quarks and leptons is that they correspond to excited states of the first generation, and experience suggests that excited systems must be composite.”
Phases of ordinary matter


In bulk, matter can exist in several different forms, or states of aggregation, known as phases, depending on ambient pressure, temperature and volume. A phase is a form of matter that has a relatively uniform chemical composition and physical properties (such as density, specific heat, refractive index, and so forth). These phases include the three familiar ones (solids, liquids, and gases), as well as more exotic states of matter ( such as plasmas, superfluids, supersolids, Bose–Einstein condensates, ...). A fluid may be a liquid, gas or plasma. There are also paramagnetic and ferromagnetic phases of magnetic materials. As conditions change, matter may change from one phase into another. These phenomena are called phase transitions, and are studied in the field of thermodynamics. In nanomaterials, the vastly increased ratio of surface area to volume results in matter that can exhibit properties entirely different from those of bulk material, and not well described by any bulk phase (see nanomaterials for more details).
Phases are sometimes called states of matter, but this term can lead to confusion with thermodynamic states. For example, two gases maintained at different pressures are in different thermodynamic states (different pressures), but in the same phase (both are gases).
Solid
Main article: SolidSolids are characterized by a tendency to retain their structural integrity; if left on their own, they will not spread in the same way gas or liquids would. Many solids, like rocks and concrete, have very high hardness and rigidity and will tend to break or shatter when subject to various forms of stress, but others like steel and paper are more flexible and will bend. Solids are often composed of crystals, glasses, or long chain molecules (e.g. rubber and paper). Some solids are amorphous such as glass. A common example of a solid is the solid form of water, ice.
Liquid
Main article: LiquidIn a liquid, the constituents frequently are touching, but able to move around each other. So unlike a gas, it has cohesion and viscosity. Compared to a solid, the forces holding constituents together are weaker, and it is not rigid, but adapts a shape decided by its container. Liquids are hard to compress. A common example is water.
Gas
Main article: GasA gas is a state of aggregation without cohesion; a vapor. Thus a gas has no resistance to changing shape (beyond the inertia of its constituents, which have to be knocked aside). The distance between constituent particles is flexible, determined, for example, by the size of a container and the number of particles, not by internal forces. A common example is the vapor form of water, steam.
Plasma
Main articles: Plasma (physics) and Astrophysical plasmaPlasma is a fourth state of matter consisting of an overall charge-neutral mix of electrons, ions and neutral atoms. The plasma exhibits behavior peculiar to long range Coulomb forces in which the particles move in electromagnetic fields generated by and self-consistent with their own motions. The sun and stars are plasmas, as is the Earth's ionosphere, and plasmas occur in neon signs. Plasmas of deuterium and tritium ions are used in fusion reactions. The term plasma was applied for the first time by Tonks and Langmuir in 1929, to the inner regions of a glowing ionized gas produced by electric discharge in a tube.
Bose–Einstein condensate
Main article: Bose–Einstein condensateThis state of matter was first discovered by Satyendra Nath Bose, who sent his work on statistics of photons to Albert Einstein for comment. Following publication of Bose's paper, Einstein extended his treatment to massive particles fixed in number, and predicted this fifth state of matter in 1925. Bose–Einstein condensates were first realized experimentally by several different scientific groups in 1995 for rubidium, sodium, and lithium, using a combination of laser and evaporative cooling. Bose–Einstein condensation for atomic hydrogen was achieved in 1998.
The Bose Einstein condensate is a liquid-like superfluid that occurs at low temperatures in which all atoms occupy the same quantum state. In low-density systems, it occurs at or below 10 K.
Fermionic condensate
Main article: Fermionic condensate See also: Superconductor and BCS theoryA fermonic condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate under similar conditions. Unlike the Bose–Einstein condensates, fermionic condensates are formed using fermions instead of bosons. The earliest recognized fermionic condensate described the state of electrons in a superconductor; the physics of other examples including recent work with fermionic atoms is analogous. The first atomic fermionic condensate was created in 2003. These atomic fermionic condensates are studied at temperatures in the vicinity of 50–350 nK.
A hypothetical fermionic condensate that appears in theories of massless fermions with chiral symmetry breaking is the chiral condensate or the quark-condensate.

Core of a neutron star
Main articles: Neutron star and Pulsar See also: MagnetarBecause of its extreme density, the core of a neutron star falls under no other state of matter. While a white dwarf is about as massive as the sun (up to 1.4 solar masses, the Chandrasekhar limit), the Pauli exclusion principle prevents its collapse to smaller radius, and it becomes an example of degenerate matter. In contrast, neutron stars are between 1.5 and 3 solar masses, and achieve such density that the protons and electrons are crushed to become neutrons. Neutrons are fermions, so further collapse is prevented by the exclusion principle, forming so-called neutron degenerate matter.

Quark–gluon plasma
Main articles: Quark–gluon plasma and QCD matter See also: Gluon and HadronGluons are elementary particles that cause quarks to interact, and are indirectly responsible for the binding of protons and neutrons together in atomic nuclei. The quark–gluon plasma is a hypothetical phase of matter, a phase of matter as yet not observed, supposed to exist in the early universe and to have evolved into a hadronic-gas phase. At extremely high energy the strong force is anticipated to become so weak that the atomic nuclei break down into a bunch of loose quarks, which distinguishes the quark–gluon phase from normal plasma. In collisions of relativistic heavy ions, a phase transition occurs from the nuclear, hadronic phase to a matter phase consisting of quarks and gluons. So far, experimental results have shown that instead of a weakly interacting plasma, an almost ideal liquid is produced. An animation can be found at Gold ion collision @ RHIC.
Transparent aluminium
Main article: Transparent aluminiumIn 2009, scientists from Oxford University led an international team in using the FLASH laser synchrotron in Hamburg, Germany to create transparent aluminum, which they described as a "new state of matter". Using a short pulse from the FLASH laser, they removed a core electron from each aluminium atom, but did not destroy or disrupt the metal’s crystalline structure. The resulting excited state of aluminium was almost transparent to extreme ultraviolet radiation. Scientists involved in the discovery suggest that this will aid in further research concerning planetary science and nuclear fusion. The effect on the aluminium lasted for 40 femtoseconds.
Structure of ordinary matter
In particle physics, fermions are particles which obey Fermi–Dirac statistics. Fermions can be elementary, like the electron, or composite, like the proton and the neutron. In the Standard Model there are two types of elementary fermions: quarks and leptons, which are discussed next.
Quarks
Main article: QuarkQuarks are a particles of spin-1⁄2, implying that they are fermions. They carry an electric charge of −1⁄3 e (down-type quarks) or +2⁄3 e (up-type quarks). For comparison, an electron has a charge of −1 e. They also carry colour charge, which is the equivalent of the electric charge for the strong interaction. Quarks also undergo radioactive decay, meaning that they are subject to the weak interaction. Quarks are massive particles, and therefore are also subject to gravity.
| name | symbol | spin | electric charge (e) |
mass (MeV/c) |
mass comparable to | antiparticle | antiparticle symbol |
|---|---|---|---|---|---|---|---|
| up-type quarks | |||||||
| up | u |
1⁄2 | +2⁄3 | 1.5 to 3.3 | ~ 5 electrons | antiup | u |
| charm | c |
1⁄2 | +2⁄3 | 1160 to 1340 | ~ 1 proton | anticharm | c |
| top | t |
1⁄2 | +2⁄3 | 169,100 to 173,300 | ~ 180 protons or ~ 1 tungsten atom |
antitop | t |
| down-type quarks | |||||||
| down | d |
1⁄2 | −1⁄3 | 3.5 to 6.0 | ~ 10 electrons | antidown | d |
| strange | s |
1⁄2 | −1⁄3 | 70 to 130 | ~ 200 electrons | antistrange | s |
| bottom | b |
1⁄2 | −1⁄3 | 4130 to 4370 | ~ 5 protons | antibottom | b |
Baryonic matter
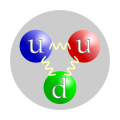
Main article: BaryonBaryons are strongly interacting fermions, and so are subject to Fermi-Dirac statistics. Amongst the baryons are the protons and neutrons, which occur in atomic nuclei, but many other unstable baryons exist as well. The term baryon is usually used to refer to triquarks — particles made of three quarks. "Exotic" baryons made of four quarks and one antiquark are known as the pentaquarks, but their existence is not generally accepted.
Baryonic matter is the part of the universe that is made of baryons (including all atoms). This part of the universe does not include dark energy, dark matter, black holes or various forms of degenerate matter, such as compose white dwarf stars and neutron stars. Microwave light seen by Wilkinson Microwave Anisotropy Probe (WMAP), suggests that only about 4.6% of that part of the universe within range of the best telescopes (that is, matter that may be visible because light could reach us from it), is made of baryionic matter. About 23% is dark matter, and about 72% is dark energy.

Degenerate matter
Main article: Degenerate matterIn physics, degenerate matter refers to the ground state of a gas of fermions at a temperature near absolute zero. The Pauli exclusion principle requires that only two fermions can occupy a quantum state, one spin-up and the other spin-down. Hence, at zero temperature, the fermions fill up sufficient levels to accommodate all the available fermions, and for the case of many fermions the maximum kinetic energy called the Fermi energy and the pressure of the gas becomes very large and dependent upon the number of fermions rather than the temperature, unlike normal states of matter.
Degenerate matter is thought to occur during the evolution of heavy stars. The demonstration by Subrahmanyan Chandrasekhar that white dwarf stars have a maximum allowed mass because of the exclusion principle caused a revolution in the theory of star evolution.
Degenerate matter includes the part of the universe that is made up of neutron stars and white dwarfs.
Strange matter
Main article: Strange matterStrange matter is a particular form of quark matter, usually thought of as a 'liquid' of up, down, and strange quarks. It is to be contrasted with nuclear matter, which is a liquid of neutrons and protons (which themselves are built out of up and down quarks), and with non-strange quark matter, which is a quark liquid containing only up and down quarks. At high enough density, strange matter is expected to be color superconducting. Strange matter is hypothesized to occur in the core of neutron stars, or, more speculatively, as isolated droplets that may vary in size from femtometers (strangelets) to kilometers (quark stars).
Two meanings of the term "strange matter"
In particle physics and astrophysics, the term is used in two ways, one broader and the other more specific.
- The broader meaning is just quark matter that contains three flavors of quarks: up, down, and strange. In this definition, there is a critical pressure and an associated critical density, and when nuclear matter (made of protons and neutrons) is compressed beyond this density, the protons and neutrons dissociate into quarks, yielding quark matter (probably strange matter).
- The narrower meaning is quark matter that is more stable than nuclear matter. The idea that this could happen is the "strange matter hypothesis" of Bodmer and Witten. In this definition, the critical pressure is zero: the true ground state of matter is always quark matter. The nuclei that we see in the matter around us, which are droplets of nuclear matter, are actually metastable, and given enough time (or the right external stimulus) would decay into droplets of strange matter, i.e. strangelets.
Leptons
Main article: LeptonLeptons are a particles of spin-1⁄2, meaning that they are fermions. They carry an electric charge of −1 e (charged leptons) or 0 e (neutrinos). Unlike quarks, leptons do not carry colour charge, meaning that they do not experience the strong interaction. Leptons also undergo radioactive decay, meaning that they are subject to the weak interaction. Leptons are massive particles, therefore are subject to gravity.
| name | symbol | spin | electric charge (e) |
mass (MeV/c) |
mass comparable to | antiparticle | antiparticle symbol |
|---|---|---|---|---|---|---|---|
| charged leptons | |||||||
| electron | e |
1⁄2 | −1 | 0.5110 | 1 electron | antielectron | e |
| muon | μ |
1⁄2 | −1 | 105.7 | ~ 200 electrons | antimuon | μ |
| tau | τ |
1⁄2 | −1 | 1,777 | ~ 2 protons | antitau | τ |
| neutrinos | |||||||
| electron neutrino | ν e |
1⁄2 | 0 | < 0.000460 | < 1⁄1000 electron | electron antineutrino | ν e |
| muon neutrino | ν μ |
1⁄2 | 0 | < 0.19 | < 1⁄2 electron | muon antineutrino | ν μ |
| tau neutrino | ν τ |
1⁄2 | 0 | < 18.2 | < 40 electrons | tau antineutrino | ν τ |
Antimatter
Main article: Antimatter Unsolved problem in physics: Baryon asymmetry. Why is there far more matter than antimatter in the observable universe? (more unsolved problems in physics)In particle physics and quantum chemistry, antimatter is matter that is composed of the antiparticles of those that constitute ordinary matter. If a particle and its antiparticle come into contact with each other, the two annihilate; that is, they may both be converted into other particles with equal energy in accordance with Einstein's equation E = mc. These new particles may be high-energy photons (gamma rays) or other particle–antiparticle pairs. The resulting particles are endowed with an amount of kinetic energy equal to the difference between the rest mass of the products of the annihilation and the rest mass of the original particle-antiparticle pair, which is often quite large.
Antimatter is not found naturally on Earth, except very briefly and in vanishingly small quantities (as the result of radioactive decay or cosmic rays). This is because antimatter which came to exist on Earth outside the confines of a suitable physics laboratory would almost instantly meet the ordinary matter that Earth is made of, and be annihilated. Antiparticles and some stable antimatter (such as antihydrogen) can be made in tiny amounts, but not in enough quantity to do more than test a few of its theoretical properties.
There is considerable speculation both in science and science fiction as to why the observable universe is apparently almost entirely matter, and whether other places are almost entirely antimatter instead. In the early universe, it is thought that matter and antimatter were equally represented, and the disappearance of antimatter requires an asymmetry in physical laws called the charge parity (or CP symmetry) violation. CP symmetry violation can be obtained from the Standard Model, but at this time the apparent asymmetry of matter and antimatter in the visible universe is one of the great unsolved problems in physics. Possible processes by which it came about are explored in more detail under baryogenesis.
Other types of matter

Ordinary matter, in the quarks and leptons definition, constitutes about 4% of the energy of the observable universe. The remaining energy is theorized to be due to exotic forms, of which 23% is dark matter and 73% is dark energy.
Dark matter
Main articles: Dark matter, Lambda-CDM model, and WIMPs See also: Galaxy formation and evolution and Dark matter haloIn astrophysics and cosmology, dark matter is matter of unknown composition that does not emit or reflect enough electromagnetic radiation to be observed directly, but whose presence can be inferred from gravitational effects on visible matter. Observational evidence of the early universe and the big bang theory require that this matter have energy and mass, but is not composed of either elementary fermions (as above) OR gauge bosons. The commonly accepted view is that most of the dark-matter is non-baryonic in nature. As such, it is composed of particles as yet unobserved in the laboratory. Perhaps they are supersymmetric particles, which are not Standard Model particles, but relics formed at very high energies in the early phase of the universe and still floating about.
Dark energy
Main article: Dark energy See also: Big bang § Dark energyIn cosmology, dark energy is the name given to the antigravitating influence that is accelerating the rate of expansion of the universe. It is known not to be composed of known particles like protons, neutrons or electrons, nor of the particles of dark matter, because these all gravitate.
Fully 70% of the matter density in the universe appears to be in the form of dark energy. Twenty-six percent is dark matter. Only 4% is ordinary matter. So less than 1 part in 20 is made out of matter we have observed experimentally or described in the standard model of particle physics. Of the other 96%, apart from the properties just mentioned, we know absolutely nothing.
— Lee Smolin: The Trouble with Physics, p. 16
Exotic matter
Main article: Exotic matterExotic matter is a hypothetical concept of particle physics. It covers any material which violates one or more classical conditions or is not made of known baryonic particles. Such materials would possess qualities like negative mass or being repelled rather than attracted by gravity.
See also
|
Antimatter
|
Cosmology
|
Dark matter
|
Philosophy Other
|
References
| Constructs such as ibid., loc. cit. and idem are discouraged by Misplaced Pages's style guide for footnotes, as they are easily broken. Please improve this article by replacing them with named references (quick guide), or an abbreviated title. (March 2010) (Learn how and when to remove this message) |
-
R. Penrose (1991). "The mass of the classical vacuum". The Philosophy of Vacuum. Oxford University Press. p. 21. ISBN 0198244495.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - "Matter (physics)". McGraw-Hill's Access Science: Encyclopedia of Science and Technology Online. Retrieved 2009-05-24.
- J. Mongillo (2007). Nanotechnology 101. Greenwood Publishing. p. 30. ISBN 0313338809.
- J. Olmsted, G.M. Williams (1996). Chemistry: The Molecular Science (2nd ed.). Jones & Bartlett. p. 40. ISBN 0815184506.
- P. Davies (1992). The New Physics: A Synthesis. Cambridge University Press. p. 1. ISBN 0521438314.
- G. 't Hooft (1997). In search of the ultimate building blocks. Cambridge University Press. p. 6. ISBN 0521578833.
- ^ "RHIC Scientists Serve Up "Perfect" Liquid" (Press release). Brookhaven National Laboratory. 18 April 2005. Retrieved 2009-09-15. Cite error: The named reference "RHIC" was defined multiple times with different content (see the help page).
- ^ P.C.W. Davies (1979). The Forces of Nature. Cambridge University Press. p. 116. ISBN 052122523X.
- ^ S. Weinberg (1998). The Quantum Theory of Fields. Cambridge University Press. p. 2. ISBN 0521550025.
- M. Masujima (2008). Path Integral Quantization and Stochastic Quantization. Springer. p. 103. ISBN 3540878505.
- ^
D. Majumdar (2007). "Dark matter — possible candidates and direct detection". arXiv:hep-ph/0703310.
{{cite arXiv}}:|class=ignored (help) Cite error: The named reference "Majumdar" was defined multiple times with different content (see the help page). - Stephen Toulmin and June Goodfield, The Architecture of Matter (Chicago: University of Chicago Press, 1962), 48-54.
- Discussed by Aristotle in Physics, esp. book I, but also later; as well as Metaphysics I-II.
- See Richard J. Connell, Matter and Becoming (Chicago: The Priory Press, 1966) for a good explanation and elaboration.
- Henry George Liddell, Robert Scott, James Morris Whiton, A lexicon abridged from Liddell & Scott's Greek-English lexicon (New York: Harper and Brothers, 1891), 725.
- René Descartes Principles of Philosophy I (1644) “The Principles of Human Knowledge,” 53.
- Ibid, 8, 54, 63.
- David L. Schindler, "The Problem of Mechanism," Beyond Mechanism, ed. David L. Schindler (University Press of America, 1986).
- ^ Newton's 31st query, as quoted by D.R. Oldroyd (1986). The Arch of Knowledge: An Introductory Study of the History of the Philosophy and Methodology of Science. Routledge. p. 83. ISBN 0416013414.
- ^ Noam Chomsky (1988). Language and problems of knowledge: the Managua lectures (2 ed.). MIT Press. p. 144. ISBN 0262530708.
- M. Wenham (2005). Understanding Primary Science: Ideas, Concepts and Explanations (2nd ed.). Paul Chapman Educational Publishing. p. 115. ISBN 1412901634.
- J.C. Maxwell (1876). Matter and Motion. Society for Promoting Christian Knowledge. p. 18. ISBN 0486668959.
- T.H. Levere (1993). "Introduction". Affinity and Matter: Elements of Chemical Philosophy, 1800–1865. Taylor & Francis. ISBN 2881245838.
- G.F. Barker (1870). "Introduction". A Text Book of Elementary Chemistry: Theoretical and Inorganic. John P. Morton and Company. p. 2.
- J.J. Thomson (1909). "Preface". Electricity and Matter. A. Constable.
- O.W. Richardson (1914). "Chapter 1". The Electron Theory of Matter. The University Press.
- M. Jacob (1992). The Quark Structure of Matter. World Scientific. ISBN 9810236875.
- V. de Sabbata, M. Gasperini (1985). Introduction to Gravitation. World Scientific. p. 293. ISBN 9971500493.
-
The history of the concept of matter is a history of the fundamental length scales used to define matter. Different building blocks apply depending upon whether one defines matter on an atomic or elementary particle level. One may use a definition that matter is atoms, or that matter is hadrons, or that matter is leptons and quarks depending upon the scale at which one wishes to define matter.
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). "Fundamental constituents of matter". Particles and Nuclei: An Introduction to the Physical Concepts (4th ed.). Springer. ISBN 3540201688.
{{cite book}}: CS1 maint: multiple names: authors list (link) - J. Allday (2001). Quarks, Leptons and the Big Bang. CRC Press. p. 12. ISBN 0750308060.
- B.A. Schumm (2004). Deep Down Things: The Breathtaking Beauty of Particle Physics. John Hopkins University Press. p. 57. ISBN 080187971X.
- See for example, M. Jibu, K. Yasue (1995). Quantum Brain Dynamics and Consciousness. John Benjamins Publishing Company. p. 62. ISBN 1556191839., B. Martin (2009). Nuclear and Particle Physics (2nd ed.). Wiley. p. 125. ISBN 0470742755. and K.W. Plaxco, M. Gross (2006). Astrobiology: A Brief Introduction. Johns Hopkins University Press. p. 23. ISBN 0801883679..
- P.A. Tipler, R.A. Llewellyn (2002). Modern Physics. Macmillan. pp. 89–91, 94–95. ISBN 0716743450.
-
P. Schmüser, H. Spitzer (2002). "Particles". In L. Bergmann; et al. (eds.). Constituents of Matter: Atoms, Molecules, Nuclei. CRC Press. pp. 773 ff. ISBN 0849312027.
{{cite book}}: Explicit use of et al. in:|editor=(help) - P.M. Chaikin, T.C. Lubensky (2000). Principles of Condensed Matter Physics. Cambridge University Press. p. xvii. ISBN 0521794501.
-
W. Greiner (2003). W. Greiner, M.G. Itkis, G. Reinhardt, M.C. Güçlü (ed.). Structure and Dynamics of Elementary Matter. Springer. p. xii. ISBN 1402024452.
{{cite book}}: CS1 maint: multiple names: editors list (link) - P. Sukys (1999). Lifting the Scientific Veil: Science Appreciation for the Nonscientist. Rowman & Littlefield. p. 87. ISBN 0847696006.
- S.M. Walker, A. King (2005). What is Matter?. Lerner Publications. p. 7. ISBN 0822551314.
-
J.Kenkel, P.B. Kelter, D.S. Hage (2000). Chemistry: An Industry-based Introduction with CD-ROM. CRC Press. p. 2. ISBN 1566703034.
All basic science textbooks define matter as simply the collective aggregate of all material substances that occupy space and have mass or weight.
{{cite book}}: CS1 maint: multiple names: authors list (link) - K.A. Peacock (2008). The Quantum Revolution: A Historical Perspective. Greenwood Publishing Group. p. 47. ISBN 031333448X.
- M.H. Krieger (1998). Constitutions of Matter: Mathematically Modeling the Most Everyday of Physical Phenomena. University of Chicago Press. p. 22. ISBN 0226453057.
- "SI brochure, Section 2.1.1.6 – Mole". BIPM. Retrieved 2009-04-30.
- M. de Podesta (2002). Understanding the Properties of Matter (2nd ed.). CRC Press. p. 8. ISBN 0415257883.
-
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). "Part I: Analysis: The building blocks of matter". Particles and Nuclei: An Introduction to the Physical Concepts (4th ed.). Springer. ISBN 3540201688.
{{cite book}}: CS1 maint: multiple names: authors list (link) - B. Carithers, P. Grannis (1995). "Discovery of the Top Quark" (PDF). Beam Line. 25 (3). SLAC: 4–16.
- See p.7 in B. Carithers, P. Grannis (1995). "Discovery of the Top Quark" (PDF). Beam Line. 25 (3). SLAC: 4–16.
- ^ D. Green (2005). High PT physics at hadron colliders. Cambridge University Press. p. 23. ISBN 0521835097. Cite error: The named reference "Green" was defined multiple times with different content (see the help page).
- L. Smolin (2007). The Trouble with Physics: The Rise of String Theory, the Fall of a Science, and What Comes Next. Mariner Books. p. 67. ISBN 061891868X.
- The W boson mass is 80.398 GeV; see Figure 1 in C. Amsler et al. (Particle Data Group) (2008). "The mass and width of the W boson" (PDF). Physics Letters B. 667: 1. Retrieved 2009-12-23.
- I.J.R. Aitchison, A.J.G. Hey (2004). Gauge Theories in Particle Physics. CRC Press. p. 48. ISBN 0750308648.
-
B. Povh, K. Rith, C. Scholz, F. Zetsche, M. Lavelle (2004). Particles and Nuclei: An Introduction to the Physical Concepts. Springer. p. 103. ISBN 3540201688=.
{{cite book}}: Check|isbn=value: invalid character (help)CS1 maint: multiple names: authors list (link) - T. Hatsuda (2008). "Quark-gluon plasma and QCD". In H. Akai (ed.). Condensed matter theories. Vol. 21. Nova Publishers. p. 296. ISBN 1600215017.
- ^ Y. Ne'eman, Y. Kirsh (1996). The particle hunters (2nd ed.). Cambridge University Press. p. 276. ISBN 0521476860.
- K.W Staley (2004). "Origins of the third generation of matter". The evidence for the top quark. Cambridge University Press. p. 8. ISBN 0521827108.
- S.R. Logan (1998). Physical Chemistry for the Biomedical Sciences. CRC Press. pp. 110–111. ISBN 0748407103.
- P.J. Collings (2002). "Chapter 1: States of Matter". Liquid Crystals: Nature's Delicate Phase of Matter. Princeton University Press. ISBN 0691086729.
- D.H. Trevena (1975). "Chapter 1.2: Changes of phase". The Liquid Phase. Taylor & Francis. ISBN 9780851090313.
- T. Makabe, Z. Petrović (2006). Plasma Electronics: Applications in Microelectronic Device Fabrication. CRC Press. p. 1. ISBN 0750309768.
- C.K. Birdsall, A.B. Langdon (2005). Plasma Physics via Computer Simulation. CRC Press. p. xvii. ISBN 0750310251.
- J.A. Bittencourt (2004). Fundamentals of Plasma Physics. Springer. p. 2. ISBN 0387209751.
- G. Fraser (2006). The New Physics for the Twenty-first Century. Cambridge University Press. p. 238. ISBN 0521816009.
- ^ C. Pethick, H. Smith (2002). "Introduction". Bose–Einstein Condensation in Dilute Gases. Cambridge University Press. ISBN 0521665809.
-
M. Greiner, C.A. Regal, S. Jin (2003). "A molecular Bose–Einstein condensate emerges from a Fermi sea". arXiv:cond-mat/0311172.
{{cite arXiv}}:|class=ignored (help)CS1 maint: multiple names: authors list (link) -
M.W. Zwierlein, C.H. Schunck, A. Schirotzek, W. Ketterle (2006). "Direct Observation of the Superfluid Phase Transition in Ultracold Fermi Gases". arXiv:cond-mat/0605258.
{{cite arXiv}}:|class=ignored (help)CS1 maint: multiple names: authors list (link) - E.V. Shuryak (2004). The QCD Vacuum, Hadrons and Superdense Matter. World Scientific. p. 159. ISBN 9812385746.
-
P. Haensel, A.Y. Potekhin, A.Û. Potehin, D.G. Yakovlev (2007). Neutron Stars. Springer. p. 11. ISBN 0387335439.
{{cite book}}: CS1 maint: multiple names: authors list (link) -
J.-P. Luminet, A. Bullough, A. King (1992). Black Holes. Cambridge University Press. p. 111, Fig. 25. ISBN 0521409063.
{{cite book}}: CS1 maint: multiple names: authors list (link) - D.R. Danielson (2001). The Book of the Cosmos. Da Capo Press. p. 455. ISBN 0738204986.
- M.A. Strain (2004). Cosmic Entity. iUniverse (self-published). p. 50. ISBN 0595301258.
- P.J. Siemens, A.S. Jensen (1994). Elements Of Nuclei: Many-body Physics With The Strong Interaction. Westview Press. p. 347. ISBN 0201627310.
- J. Letessier, J. Rafelski (2002). Hadrons and Quark–Gluon Plasma. Cambridge University Press. p. xi. ISBN 0521385369.
- W.A. Zajc (2008). "The fluid nature of quark–gluon plasma". Nuclear Physics A. 805: 283c – 294c. doi:10.1016/j.nuclphysa.2008.02.285. arXiv:0802.3552.
- "Transparent Aluminium Is 'New State Of Matter'". Science Daily. 27 July 2009. Retrieved 2009-07-30.
-
C. Amsler et al. (Particle Data Group) (2008). Physics Letters B. 667: 1.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|link=ignored (help) - "Five Year Results on the Oldest Light in the Universe". NASA. 2008. Retrieved 2008-05-02.
- H.S. Goldberg, M.D. Scadron (1987). Physics of Stellar Evolution and Cosmology. Taylor & Francis. p. 202. ISBN 0677055404.
- H.S. Goldberg, M.D. Scadron (1987). Physics of Stellar Evolution and Cosmology. Taylor & Francis. p. 233. ISBN 0677055404.
-
J.-P. Luminet, A. Bullough, A. King (1992). Black Holes. Cambridge University Press. p. 75. ISBN 0521409063.
{{cite book}}: CS1 maint: multiple names: authors list (link) - A. Bodmer (1971). "Collapsed Nuclei". Physical Review D. 4 (6): 1601. doi:10.1103/PhysRevD.4.1601.
- E. Witten (1984). "Cosmic Separation of Phases". Physical Review D. 30 (2): 272. doi:10.1103/PhysRevD.30.272.
-
C. Amsler et al. (Particle Data Group) (2008). Physics Letters B. 667: 1.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|link=ignored (help) -
C. Amsler et al. (Particle Data Group) (2008). Physics Letters B. 667: 1.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|link=ignored (help) - National Research Council (US) (2006). Revealing the hidden nature of space and time. National Academies Press. p. 46. ISBN 0309101948.
-
J.P. Ostriker, P.J. Steinhardt (2003). "New Light on Dark Matter". arXiv:astro-ph/0306402.
{{cite arXiv}}:|class=ignored (help) - K. Pretzl (2004). "Dark Matter, Massive Neutrinos and Susy Particles". Structure and Dynamics of Elementary Matter. Walter Greiner. p. 289. ISBN 1402024460.
- K. Freeman, G. McNamara (2006). "What can the matter be?". In Search of Dark Matter. Birkhäuser. p. 105. ISBN 0387276165.
- J.C. Wheeler (2007). Cosmic Catastrophes: Exploding Stars, Black Holes, and Mapping the Universe. Cambridge University Press. p. 282. ISBN 0521857147.
- J. Gribbin (2007). The Origins of the Future: Ten Questions for the Next Ten Years. Yale University Press. p. 151. ISBN 0300125968.
- P. Schneider (2006). Extragalactic Astronomy and Cosmology. Springer. p. 4, Fig. 1.4. ISBN 3540331743.
- T. Koupelis, K.F. Kuhn (2007). In Quest of the Universe. Jones & Bartlett Publishers. p. 492; Fig. 16.13. ISBN 0763743879.
-
M.H. Jones, R.J. Lambourne, D.J. Adams (2004). An Introduction to Galaxies and Cosmology. Cambridge University Press. p. 21; Fig. 1.13. ISBN 0521546230.
{{cite book}}: CS1 maint: multiple names: authors list (link) -
K.A. Olive (2003). "Theoretical Advanced Study Institute lectures on dark matter". arXiv:astro-ph/0301505.
{{cite arXiv}}:|class=ignored (help) - K.A. Olive (2009). "Colliders and Cosmology". European Physical Journal C. 59: 269–295. doi:10.1140/epjc/s10052-008-0738-8. arXiv:0806.1208.
- J.C. Wheeler (2007). Cosmic Catastrophes. Cambridge University Press. p. 282. ISBN 0521857147.
- L. Smolin (2007). The Trouble with Physics. Mariner Books. p. 16. ISBN 061891868X.
Further reading
- Lillian Hoddeson, Michael Riordan, ed. (1997). The Rise of the Standard Model. Cambridge University Press. ISBN 0521578167.
- Timothy Paul Smith (2004). "The search for quarks in ordinary matter". Hidden Worlds. Princeton University Press. p. 1. ISBN 0691057737.
- Harald Fritzsch (2005). Elementary Particles: Building blocks of matter. World Scientific. p. 1. ISBN 9812561412.
- Bertrand Russell (1992). "The philosophy of matter". A Critical Exposition of the Philosophy of Leibniz (Reprint of 1937 2nd ed.). Routledge. p. 88. ISBN 041508296X.
- Stephen Toulmin and June Goodfield, The Architecture of Matter (Chicago: University of Chicago Press, 1962).
- Richard J. Connell, Matter and Becoming (Chicago: The Priory Press, 1966).
- Ernan McMullin, The Concept of Matter in Greek and Medieval Philosophy (Notre Dame, IN: Univ. of Notre Dame Press, 1965).
- Ernan McMullin, The Concept of Matter in Modern Philosophy (Notre Dame, IN: University of Notre Dame Press, 1978).
External links
- Visionlearning Module on Matter
- Matter in the universe How much Matter is in the Universe?
- NASA on superfluid core of neutron star
| States of matter (list) | ||
|---|---|---|
| State |  | |
| Low energy | ||
| High energy | ||
| Other states | ||
| Phase transitions |
| |
| Quantities | ||
| Concepts | ||
| Particles in physics | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elementary |
| ||||||||||||||||||||||||
| Composite |
| ||||||||||||||||||||||||
| Quasiparticles | |||||||||||||||||||||||||
| Lists | |||||||||||||||||||||||||
| Related | |||||||||||||||||||||||||