| Revision as of 07:08, 17 September 2012 editBr'er Rabbit (talk | contribs)8,858 edits cite book 978-0-340-66316-5 — prolly needs a chapter specified…← Previous edit | Revision as of 07:24, 17 September 2012 edit undoBr'er Rabbit (talk | contribs)8,858 edits de-clutter named refs; more of this when <ref> have names…Next edit → | ||
| Line 4: | Line 4: | ||
| | author = ] | | author = ] | ||
| | developer = Pande laboratory, ], ], ], Cauldron Development<ref name="about" /> | | developer = Pande laboratory, ], ], ], Cauldron Development<ref name="about" /> | ||
| | released = October 1, 2000<ref name="FoldingFAQ" /> | |||
| | released = October 1, 2000<ref>{{cite web | url = http://www.stanford.edu/group/pandegroup/folding/FoldingFAQ.pdf | title = Folding@Home Executive summary | author = Pande lab | work = Folding@home | publisher = ] | accessdate = October 4, 2011 }}</ref> | |||
| | latest release version = ''Windows'', ''Linux'': 7.1.52<ref name="FAH homepage" /><br/> ''Mac OS X:'' 6.29.3<ref name="FAH homepage" /><br/> ''PlayStation 3'': 1.4<ref |
| latest release version = ''Windows'', ''Linux'': 7.1.52<ref name="FAH homepage" /><br/> ''Mac OS X:'' 6.29.3<ref name="FAH homepage" /><br/> ''PlayStation 3'': 1.4<ref name="FAH 4 PS3" /> | ||
| | operating system = ], ], ] | | operating system = ], ], ] | ||
| | platform = ] | | platform = ] | ||
| Line 14: | Line 14: | ||
| }} | }} | ||
| '''Folding@home''' ('''FAH''' or '''F@h''') is a ] project for simulation of ], computational ], and other ] for disease research. Folding@home is powered by the idle processing resources of thousands of ] and ]s from volunteers who have installed the software on these systems. The project primarily attempts to determine the mechanisms of ], (the process by which ]s reach their ]) and the reasons behind ]. This is of significant academic interest and has major implications for ] into ], ], and many forms of cancer, among other diseases. To a lesser extent, Folding@home also tries to ] a protein's ] and determine how other molecules may ] with it, which has applications in drug design. Folding@home is developed and operated by the Pande laboratory at ], under the leadership of ], and is shared by various scientific institutions and research laboratories across the world in a collaboration known as the Folding@home Consortium.<ref name="about" |
'''Folding@home''' ('''FAH''' or '''F@h''') is a ] project for simulation of ], computational ], and other ] for disease research. Folding@home is powered by the idle processing resources of thousands of ] and ]s from volunteers who have installed the software on these systems. The project primarily attempts to determine the mechanisms of ], (the process by which ]s reach their ]) and the reasons behind ]. This is of significant academic interest and has major implications for ] into ], ], and many forms of cancer, among other diseases. To a lesser extent, Folding@home also tries to ] a protein's ] and determine how other molecules may ] with it, which has applications in drug design. Folding@home is developed and operated by the Pande laboratory at ], under the leadership of ], and is shared by various scientific institutions and research laboratories across the world in a collaboration known as the Folding@home Consortium.<ref name="about" /> | ||
| The project uses statistical simulation methodology that represents a ] from traditional computational approaches.<ref name="Everything about MSMs" |
The project uses statistical simulation methodology that represents a ] from traditional computational approaches.<ref name="Everything about MSMs" /> As part of the project's ] ], the volunteered machines receive simulation Work Units, complete them, and return them to ]s where they are compiled into an overall simulation. Volunteers can track their contributions on the Folding@home website, which can make participation competitive and encourages long-term involvement. The project has pioneered the uses of ]s, PlayStation 3s, and ] (used for computing on ]s) for distributed computing and scientific research. | ||
| Folding@home remains one of the world's fastest computing systems, and currently operates at a computational performance nearly equal to all distributed computing projects under ] combined. The project is also the world's most powerful molecular dynamics simulator. This performance from its large-scale computing network has allowed researchers to run ] atomic-level simulations thousands of times longer than previously achieved. Since its launch on October 1, 2000, the Pande lab has produced 100 ] as a direct result of the project.<ref name="papers" |
Folding@home remains one of the world's fastest computing systems, and currently operates at a computational performance nearly equal to all distributed computing projects under ] combined. The project is also the world's most powerful molecular dynamics simulator. This performance from its large-scale computing network has allowed researchers to run ] atomic-level simulations thousands of times longer than previously achieved. Since its launch on October 1, 2000, the Pande lab has produced 100 ] as a direct result of the project.<ref name="papers" /> These simulations have demonstrated accuracy compared to experimental observations.<ref name="NTL9 folding" /><ref>{{cite journal | author = Gregory R. Bowman and Vijay S. Pande | title = Protein folded states are kinetic hubs | journal = Proceedings of the National Academy of Sciences | year = 2010 | volume = 107 | issue = 24 | page = 10890 | doi = 10.1073/pnas.1003962107 | bibcode = 2010PNAS..10710890B }}</ref> | ||
| == Project significance == | == Project significance == | ||
| Line 24: | Line 24: | ||
| ] | ] | ||
| ]s are an essential component to many biological functions and participate in virtually all processes within ]s. They often act as ], performing biochemical reactions including ], molecular transportation, and ]. As structural elements, some proteins act as a type of ], and as ], other proteins participate in the ]. Before a protein can take on these roles, it must fold into a functional ], a process that often occurs spontaneously and is dependent on interactions within its amino acid sequence. Protein folding is driven by the search to find the most energetically favorable conformation of the protein, ''i.e.'' its ]. Thus, understanding protein folding is critical to understanding what a protein does and how it works, and is considered a "holy grail" of ].<ref>{{cite journal | author = Fabrizio Marinelli, Fabio Pietrucci, Alessandro Laio, Stefano Piana | title = A Kinetic Model of Trp-Cage Folding from Multiple Biased Molecular Dynamics Simulations | journal = PLoS Computational Biology | year = 2009 | volume = 8 | issue = 5 | pages = e1000452 | doi = 10.1371/journal.pcbi.1000452 | editor1-first = Vijay S | editor1-last = Pande }}</ref><ref>{{cite journal | title = So Much More to Know | journal = Science | year = 2005 | volume = 309 | issue = 5731 | pages = 78–102 | doi = 10.1126/science.309.5731.78b | pmid = 15994524 }}</ref> Despite folding occurring within a ], it typically proceeds smoothly. However, due to a protein's chemical properties or other factors, proteins may misfold — that is, fold down the wrong pathway and end up misshapen. Unless cellular mechanisms are capable of destroying or refolding such misfolded proteins, they can subsequently ] and cause a variety of debilitating diseases.<ref name="Unraveling mysteries of PF" |
]s are an essential component to many biological functions and participate in virtually all processes within ]s. They often act as ], performing biochemical reactions including ], molecular transportation, and ]. As structural elements, some proteins act as a type of ], and as ], other proteins participate in the ]. Before a protein can take on these roles, it must fold into a functional ], a process that often occurs spontaneously and is dependent on interactions within its amino acid sequence. Protein folding is driven by the search to find the most energetically favorable conformation of the protein, ''i.e.'' its ]. Thus, understanding protein folding is critical to understanding what a protein does and how it works, and is considered a "holy grail" of ].<ref>{{cite journal | author = Fabrizio Marinelli, Fabio Pietrucci, Alessandro Laio, Stefano Piana | title = A Kinetic Model of Trp-Cage Folding from Multiple Biased Molecular Dynamics Simulations | journal = PLoS Computational Biology | year = 2009 | volume = 8 | issue = 5 | pages = e1000452 | doi = 10.1371/journal.pcbi.1000452 | editor1-first = Vijay S | editor1-last = Pande }}</ref><ref>{{cite journal | title = So Much More to Know | journal = Science | year = 2005 | volume = 309 | issue = 5731 | pages = 78–102 | doi = 10.1126/science.309.5731.78b | pmid = 15994524 }}</ref> Despite folding occurring within a ], it typically proceeds smoothly. However, due to a protein's chemical properties or other factors, proteins may misfold — that is, fold down the wrong pathway and end up misshapen. Unless cellular mechanisms are capable of destroying or refolding such misfolded proteins, they can subsequently ] and cause a variety of debilitating diseases.<ref name="Unraveling mysteries of PF" /> Laboratory experiments studying these processes can be limited in scope and atomic detail, leading scientists to use physics-based computational models that, when complementing experiments, seek to provide a more complete picture of protein folding, misfolding, and aggregation.<ref name="PF then and now" /><ref name="from the TT to organism" /> | ||
| Due to the complexity of proteins' ] and limitations in computational power, all-atom molecular dynamics simulations have been severely limited in the timescales which they can study. While most proteins typically fold in the order of milliseconds,<ref name="PF then and now" /><ref name="how well can simulation predict" |
Due to the complexity of proteins' ] and limitations in computational power, all-atom molecular dynamics simulations have been severely limited in the timescales which they can study. While most proteins typically fold in the order of milliseconds,<ref name="PF then and now" /><ref name="how well can simulation predict" /> prior to 2010 simulations could only reach nanosecond to microsecond timescales.<ref name="NTL9 folding" /> General-purpose ]s have been used to simulate protein folding, but such systems are intrinsically expensive and typically shared between many different research groups, and because the computations in kinetic models are serial in nature, strong ] of traditional molecular simulations to these architectures is exceptionally difficult.<ref>{{cite journal | author = A. Verma, S.M. Gopal, A. Schug, J.S. Oh, K.V. Klenin, K.H. Lee, and W. Wenzel | title = Massively Parallel All Atom Protein Folding in a Single Day | journal = Advances in Parallel Computing | year = 2008 | volume = 15 | pages = 527–534 | issn = 0927-5452 | isbn = 978-1-58603-796-3 }}</ref><ref>{{cite journal | author = Vijay S. Pande, Ian Baker, Jarrod Chapman, Sidney P. Elmer, Siraj Khaliq, Stefan M. Larson, Young Min Rhee, Michael R. Shirts, Christopher D. Snow, Eric J. Sorin, Bojan Zagrovic | title = Atomistic protein folding simulations on the submillisecond timescale using worldwide distributed computing | journal = Biopolymers | year = 2002 | volume = 68 | issue = 1 | pages = 91–109 | doi = 10.1002/bip.10219 | pmid = 12579582 }}</ref> Additionally, as the protein folding process is ], a limited number of long simulations are not sufficient for comprehensive views of protein folding.<ref name="taming folding complexity" /> | ||
| Protein folding does not occur in a single step.<ref name="Unraveling mysteries of PF" /> Instead, proteins spend the majority of their folding time |
Protein folding does not occur in a single step.<ref name="Unraveling mysteries of PF" /> Instead, proteins spend the majority of their folding time – nearly 96% in some cases<ref>{{cite journal | author = Robert B Best | title = Atomistic molecular simulations of protein folding | journal = Current Opinion in Structural Biology | year = 2012 | format = review | volume = 22 | issue = 1 | pages = 52–61 | doi = 10.1016/j.sbi.2011.12.001 | pmid = 22257762 }}</ref> – "waiting" in various intermediate ] states, each a local ] minimum in the protein's ]. Through a process known as adaptive sampling, these conformations are used by Folding@home as starting points for a ] of simulations trajectories. As the simulations discover more conformations, the trajectories are restarted from them, and a ] (MSM) is gradually created from this cyclic process. MSMs are ] ] models which map out a biomolecule's conformational and energy landscape by describing its set of distinct structures and the transition rates between them. The adaptive sampling Markov state model approach significantly increases the efficiency of simulation as it avoids computation inside the local energy minimum itself, and is amenable to distributed computing (including on ]) as it allows for the statistical aggregation of short, independent simulation trajectories.<ref name="Simulation FAQ" /> The amount of time it takes to construct a Markov state model is inversely proportional to the number of parallel simulations run, ''i.e.'' the number of processors available. In other words, it achieves near-linear ], leading to an approximately four orders of magnitude reduction in overall serial calculation time. A completed MSM illustrates the probability of folding events and pathways from the protein's ], may contain tens of thousands of states, and through kinetic clustering of the conformations it can represent these states at an arbitrary resolution. Researchers can use these MSMs to reveal how proteins misfold and to quantitatively compare simulations with experiments.<ref name="Everything about MSMs" /><ref name="taming folding complexity" /><ref name="adaptive sampling of MSMs" /> Between 2000 and 2010, the timescales over which Folding@home simulates protein folding have increased by six orders of magnitude.<ref>{{cite web | url = http://folding.typepad.com/news/2012/06/fahcon-2012-thinking-about-how-far-fah-has-come.html | title = FAHcon 2012: Thinking about how far FAH has come | author = Vijay Pande | work = Folding@home | publisher = ] | date = June 8, 2012 | accessdate = June 12, 2012 }}</ref> | ||
| In 2002, Folding@home used Markov state models to complete approximately a million CPU days of simulations over the span of several months,<ref>{{cite journal | author = Christopher D. Snow, Houbi Ngyen, Vijay S. Pande, and Martin Gruebele | title = Absolute comparison of simulated and experimental protein-folding dynamics | journal = Nature | year = 2002 | volume = 420 | issue = 6911 | pages = 102–106 | doi = 10.1038/nature01160 | pmid = 12422224 | url = http://sansan.phy.ncu.edu.tw/~hclee/ref/SnowNature2002.pdf | bibcode = 2002Natur.420..102S }}</ref> and in 2011, MSMs parallelized another simulation that required an aggregate 10 million CPU hours of computation.<ref>{{cite journal | author = Kyle A. Beauchamp, Daniel L. Ensign, Rhiju Das, and Vijay S. Pande | title = Quantitative comparison of villin headpiece subdomain simulations and triplet–triplet energy transfer experiments | journal = Proceedings of the National Academy of Sciences | year = 2011 | volume = 108 | issue = 31 | page = 12734 | doi = 10.1073/pnas.1010880108 | bibcode = 2011PNAS..10812734B }}</ref> In January 2010, Folding@home used MSMs to simulate the dynamics of the slow-folding 32-] NTL9 protein out to 1.52 milliseconds, a timescale consistent with experimental folding rate predictions but a thousand times longer than previously achieved. The model consisted of many individual trajectories, each two orders of magnitude shorter.<ref name="Everything about MSMs" /><ref name="NTL9 folding" /> This was the first demonstration that MSMs were capable of statistically capturing folding events that could not be seen by conventional simulation methods.<ref name="taming folding complexity" /> In 2010, Folding@home researcher Greg Bowman was awarded the ] Paradigm Shift Award from the ] for the instrumental development of the ] MSMBuilder software and for attaining quantitative agreement between theory and experiment.<ref>{{cite web | url = https://simtk.org/project/xml/news.xml?group_id=357 | title = Greg Bowman awarded the 2010 Kuhn Paradigm Shift Award | work = simtk.org | publisher = SimTK: MSMBuilder | date = March 29, 2010 | accessdate = April 5, 2012 }}</ref> For his work, Pande was awarded the 2012 Michael and Kate Bárány Award for Young Investigators for "developing field-defining and field-changing computational methods to produce leading theoretical models for protein and ] folding"<ref>{{cite web | url = http://www.biophysics.org/LinkClick.aspx?fileticket=k_JYSLGevzU%3d&tabid=504 | title = Biophysical Society Names Five 2012 Award Recipients | work = Biophysics.org | publisher = Biophysical Society | date = August 17, 2011 | accessdate = April 5, 2012 }}</ref> as well as the 2006 Irving Sigal Young Investigator Award "for his unique approach to employing advances in algorithms that make optimal use of distributed computing, which places his efforts at the cutting edge of simulations. The results have stimulated a re-examination of the meaning of both ensemble and single-molecule measurements, making Dr. Pande’s efforts pioneering contributions to simulation methodology."<ref>{{cite web | url = http://folding.stanford.edu/English/Awards | title = Folding@home |
In 2002, Folding@home used Markov state models to complete approximately a million CPU days of simulations over the span of several months,<ref>{{cite journal | author = Christopher D. Snow, Houbi Ngyen, Vijay S. Pande, and Martin Gruebele | title = Absolute comparison of simulated and experimental protein-folding dynamics | journal = Nature | year = 2002 | volume = 420 | issue = 6911 | pages = 102–106 | doi = 10.1038/nature01160 | pmid = 12422224 | url = http://sansan.phy.ncu.edu.tw/~hclee/ref/SnowNature2002.pdf | bibcode = 2002Natur.420..102S }}</ref> and in 2011, MSMs parallelized another simulation that required an aggregate 10 million CPU hours of computation.<ref>{{cite journal | author = Kyle A. Beauchamp, Daniel L. Ensign, Rhiju Das, and Vijay S. Pande | title = Quantitative comparison of villin headpiece subdomain simulations and triplet–triplet energy transfer experiments | journal = Proceedings of the National Academy of Sciences | year = 2011 | volume = 108 | issue = 31 | page = 12734 | doi = 10.1073/pnas.1010880108 | bibcode = 2011PNAS..10812734B }}</ref> In January 2010, Folding@home used MSMs to simulate the dynamics of the slow-folding 32-] NTL9 protein out to 1.52 milliseconds, a timescale consistent with experimental folding rate predictions but a thousand times longer than previously achieved. The model consisted of many individual trajectories, each two orders of magnitude shorter.<ref name="Everything about MSMs" /><ref name="NTL9 folding" /> This was the first demonstration that MSMs were capable of statistically capturing folding events that could not be seen by conventional simulation methods.<ref name="taming folding complexity" /> In 2010, Folding@home researcher Greg Bowman was awarded the ] Paradigm Shift Award from the ] for the instrumental development of the ] MSMBuilder software and for attaining quantitative agreement between theory and experiment.<ref>{{cite web | url = https://simtk.org/project/xml/news.xml?group_id=357 | title = Greg Bowman awarded the 2010 Kuhn Paradigm Shift Award | work = simtk.org | publisher = SimTK: MSMBuilder | date = March 29, 2010 | accessdate = April 5, 2012 }}</ref> For his work, Pande was awarded the 2012 Michael and Kate Bárány Award for Young Investigators for "developing field-defining and field-changing computational methods to produce leading theoretical models for protein and ] folding"<ref>{{cite web | url = http://www.biophysics.org/LinkClick.aspx?fileticket=k_JYSLGevzU%3d&tabid=504 | title = Biophysical Society Names Five 2012 Award Recipients | work = Biophysics.org | publisher = Biophysical Society | date = August 17, 2011 | accessdate = April 5, 2012 }}</ref> as well as the 2006 Irving Sigal Young Investigator Award "for his unique approach to employing advances in algorithms that make optimal use of distributed computing, which places his efforts at the cutting edge of simulations. The results have stimulated a re-examination of the meaning of both ensemble and single-molecule measurements, making Dr. Pande’s efforts pioneering contributions to simulation methodology."<ref>{{cite web | url = http://folding.stanford.edu/English/Awards | title = Folding@home – Awards | work = Folding@home | publisher = ] | date = June 2006 | accessdate = August 31, 2012 }}</ref> | ||
| == Biomedical research == | == Biomedical research == | ||
| Protein folding is naturally tightly regulated to ensure that it proceeds smoothly. The failure of a protein to fold correctly can result in the development of a ] including ], Alzheimer's disease, ],<ref>{{cite journal | author = Antonella De Jaco, Michael Z. Lin, Noga Dubi, Davide Comoletti, Meghan T. Miller, Shelley Camp, Mark Ellisman, Margaret T. Butko, Roger Y. Tsien, and Palmer Taylor | title = Neuroligin Trafficking Deficiencies Arising from Mutations in the a/ß-Hydrolase Fold Protein Family | journal = Journal of Biological Chemistry | year = 2010 | volume = 285 | issue = 37 | pages = 28674–28682 | doi = 10.1074/jbc.M110.139519 | pmid = 20615874 | pmc = 2937894 }}</ref> ], ], ], ], ], ], ], and ].<ref name="Unraveling mysteries of PF" /><ref name="Protein Misfolding Diseases" |
Protein folding is naturally tightly regulated to ensure that it proceeds smoothly. The failure of a protein to fold correctly can result in the development of a ] including ], Alzheimer's disease, ],<ref>{{cite journal | author = Antonella De Jaco, Michael Z. Lin, Noga Dubi, Davide Comoletti, Meghan T. Miller, Shelley Camp, Mark Ellisman, Margaret T. Butko, Roger Y. Tsien, and Palmer Taylor | title = Neuroligin Trafficking Deficiencies Arising from Mutations in the a/ß-Hydrolase Fold Protein Family | journal = Journal of Biological Chemistry | year = 2010 | volume = 285 | issue = 37 | pages = 28674–28682 | doi = 10.1074/jbc.M110.139519 | pmid = 20615874 | pmc = 2937894 }}</ref> ], ], ], ], ], ], ], and ].<ref name="Unraveling mysteries of PF" /><ref name="Protein Misfolding Diseases" /><ref name="diseases FAQ" /> Once it is understood how a protein misfolds, ] intervention can follow, which can use engineered molecules to alter the production of a certain protein, to help destroy a misfolded protein, or to assist in the folding process.<ref>{{cite journal | author = Fred E. Cohen & Jeffery W. Kelly | title = Therapeutic approaches to protein misfolding diseases | format = review | journal = Nature | year = 2003 | volume = 426 | issue = 6968 | pages = 905–9 | doi = 10.1038/nature02265 | pmid = 14685252 }}</ref> Cellular infection by viruses such as ] and ] also involve folding events within cellular membranes.<ref name="Collier Virology" /> Computer-assisted ] has the potential to expedite and lower the costs of drug discovery.<ref name="Comp-aided drug design" /> The combination of computational molecular modeling and experimental analysis has the possibility of fundamentally shaping the future of molecular medicine and the rational design of therapeutics.<ref name="from the TT to organism" /> Folding@home is dedicated to producing significant amounts of results about protein folding, the diseases that result from protein misfolding, and the development of novel computational methods for drug design.<ref name="diseases FAQ" /> The goal of the first five years of the project was to make significant advances in understanding folding, while the current goal is to understand misfolding and related disease, especially Alzheimer's disease.<ref name="Press FAQ" /> | ||
| The simulations run on Folding@home used in conjunction with laboratory experiments,<ref name="taming folding complexity" /> but researchers can use it to study how folding '']'' differs from folding in native ] environments. This is advantageous in studying aspects of folding, misfolding, and its relationship to disease that are exceptionally difficult to observe experimentally. For example, in 2011 Folding@home continued simulations of folding inside a ] exit tunnel, to help scientists better understand how natural confinement and crowding might influence the folding process.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=24&t=19376&start=0#p193378 | title = Projects 7808 and 7809 to full fah | author = Christian "schwancr" Schwantes (Pande lab member) | work = Folding@home | publisher = ] Group | date = August 15, 2011 | accessdate = October 16, 2011 }}</ref><ref>{{cite journal | author = Del Lucent, V. Vishal, and Vijay S. Pande | title = Protein folding under confinement: A role for solvent | journal = ] | year = 2007 | volume = 104 | issue = 25 | pages = 10430–10434 | doi = 10.1073/pnas.0608256104 | url = http://www.pnas.org/content/104/25/10430.full | bibcode = 2007PNAS..10410430L }}</ref> Furthermore, scientists typically employ chemical ] to unfold proteins from their stable ]. It is not generally known how the denaturant affects the protein's refolding, and it is difficult to experimentally determine if these denatured states contain residual structures which may influence folding behavior. In 2010, Folding@home simulated the unfolded states of ], and predicted the collapse rate in strong agreement with experimental results.<ref>{{cite journal | author = Vincent A. Voelz, Vijay R. Singh, William J. Wedemeyer, Lisa J. Lapidus, and Vijay S. Pande | title = Unfolded-State Dynamics and Structure of Protein L Characterized by Simulation and Experiment | journal = Journal of the American Chemical Society | year = 2010 | volume = 132 | issue = 13 | pages = 4702–4709 | doi = 10.1021/ja908369h | pmid = 20218718 | pmc = 2853762 }}</ref> | The simulations run on Folding@home used in conjunction with laboratory experiments,<ref name="taming folding complexity" /> but researchers can use it to study how folding '']'' differs from folding in native ] environments. This is advantageous in studying aspects of folding, misfolding, and its relationship to disease that are exceptionally difficult to observe experimentally. For example, in 2011 Folding@home continued simulations of folding inside a ] exit tunnel, to help scientists better understand how natural confinement and crowding might influence the folding process.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=24&t=19376&start=0#p193378 | title = Projects 7808 and 7809 to full fah | author = Christian "schwancr" Schwantes (Pande lab member) | work = Folding@home | publisher = ] Group | date = August 15, 2011 | accessdate = October 16, 2011 }}</ref><ref>{{cite journal | author = Del Lucent, V. Vishal, and Vijay S. Pande | title = Protein folding under confinement: A role for solvent | journal = ] | year = 2007 | volume = 104 | issue = 25 | pages = 10430–10434 | doi = 10.1073/pnas.0608256104 | url = http://www.pnas.org/content/104/25/10430.full | bibcode = 2007PNAS..10410430L }}</ref> Furthermore, scientists typically employ chemical ] to unfold proteins from their stable ]. It is not generally known how the denaturant affects the protein's refolding, and it is difficult to experimentally determine if these denatured states contain residual structures which may influence folding behavior. In 2010, Folding@home simulated the unfolded states of ], and predicted the collapse rate in strong agreement with experimental results.<ref>{{cite journal | author = Vincent A. Voelz, Vijay R. Singh, William J. Wedemeyer, Lisa J. Lapidus, and Vijay S. Pande | title = Unfolded-State Dynamics and Structure of Protein L Characterized by Simulation and Experiment | journal = Journal of the American Chemical Society | year = 2010 | volume = 132 | issue = 13 | pages = 4702–4709 | doi = 10.1021/ja908369h | pmid = 20218718 | pmc = 2853762 }}</ref> | ||
| The Pande lab is a ] and does not sell the results generated by Folding@home.<ref name="Main FAQ" /> The large data sets from the project are freely available for other researchers to use upon request and some can be accessed from the Folding@home website.<ref name="F@H & Simbios" |
The Pande lab is a ] and does not sell the results generated by Folding@home.<ref name="Main FAQ" /> The large data sets from the project are freely available for other researchers to use upon request and some can be accessed from the Folding@home website.<ref name="F@H & Simbios" /><ref name="papers for free" /> The Pande lab has collaborated with other molecular dynamics systems such as the ] supercomputer,<ref name="biologists think bigger" /> and they share Folding@home's key software with other researchers, so that the algorithms which benefited Folding@home may aid other scientific areas.<ref name="F@H & Simbios" /> In 2011 they released the ] Copernicus software, which is based on Folding@home's MSM and other parallelization techniques and aims to significantly improve the efficiency and scaling of molecular simulations on large ]s or supercomputers.<ref>{{cite journal | author = S. Pronk, P. Larsson, I. Pouya, G.R. Bowman, I.S. Haque, K. Beauchamp, B. Hess, V.S. Pande, P.M. Kasson, E. Lindahl | title = Copernicus: A new paradigm for parallel adaptive molecular dynamics | journal = 2011 International Conference for High Performance Computing, Networking, Storage and Analysis | year = 2011 | pages = 1–10, 12–18 }}</ref> Summaries of all of the scientific findings from Folding@home are posted on the Folding@home website after publication.<ref name="papers" /> The full publications are available online from an academic library.<ref name="papers for free" /> | ||
| === Alzheimer's disease === | === Alzheimer's disease === | ||
| ] and cut it into Aβ fragments, which then aggregate to form ] characteristic of Alzheimer's patients.]] | ] and cut it into Aβ fragments, which then aggregate to form ] characteristic of Alzheimer's patients.]] | ||
| ] is an incurable ] disease which most most often affects the elderly. It accounts for more than half of all cases of ], and as of 2008 it affects more than 24 million people worldwide, with 4.6 million new cases reported each year. Its exact cause remains unknown, but the disease is identified as a ] and is associated with toxic ] of the ] (Aβ) ], a fragment of the larger ]. High concentrations of misfolded Aβ<sub>42</sub> causes protein ] growth leading to aggregation that in turn contributes to Aβ misfolding. This cyclic process appears to be toxic and leads to neuronal cell death. The oligomer aggregates then collect into dense nontoxic formations known as ], a pathological marker of Alzheimer's disease.<ref>{{cite journal | author = G Brent Irvine, Omar M El-Agnaf, Ganesh M Shankar, and Dominic M Walsh | title = Protein Aggregation in the Brain: The Molecular Basis for Alzheimer's and Parkinson's Diseases | journal = Molecular Medicine | format = review | volume = 14 | issue = 7–8 | pages = 451–464 | year = 2008 | pmid = 18368143 | doi = 10.2119/2007-00100.Irvine | pmc = 2274891 }}</ref><ref name="PM&N review" |
] is an incurable ] disease which most most often affects the elderly. It accounts for more than half of all cases of ], and as of 2008 it affects more than 24 million people worldwide, with 4.6 million new cases reported each year. Its exact cause remains unknown, but the disease is identified as a ] and is associated with toxic ] of the ] (Aβ) ], a fragment of the larger ]. High concentrations of misfolded Aβ<sub>42</sub> causes protein ] growth leading to aggregation that in turn contributes to Aβ misfolding. This cyclic process appears to be toxic and leads to neuronal cell death. The oligomer aggregates then collect into dense nontoxic formations known as ], a pathological marker of Alzheimer's disease.<ref>{{cite journal | author = G Brent Irvine, Omar M El-Agnaf, Ganesh M Shankar, and Dominic M Walsh | title = Protein Aggregation in the Brain: The Molecular Basis for Alzheimer's and Parkinson's Diseases | journal = Molecular Medicine | format = review | volume = 14 | issue = 7–8 | pages = 451–464 | year = 2008 | pmid = 18368143 | doi = 10.2119/2007-00100.Irvine | pmc = 2274891 }}</ref><ref name="PM&N review" /><ref name="AB assembly and AD" /> Due to the heterogeneous nature of Aβ oligomer aggregates, experimental techniques such as ] and ] have had difficulty characterizing their structures. Moreover, atomistic simulations are extremely computationally demanding due to their size and complexity.<ref name="Simulating oligomerization" /><ref name="Novick2011" /> | ||
| Disabling Aβ aggregation using small molecules is regarded as a promising approach to the development of therapeutic drugs for treating Alzheimer's patients.<ref>{{cite journal | author = Aabgeena Naeem and Naveed Ahmad Fazili | title = Defective Protein Folding and Aggregation as the Basis of Neurodegenerative Diseases: The Darker Aspect of Proteins | format = review | journal = Cell Biochemistry and Biophysics | year = 2011 | volume = 61 | issue = 2 | pages = 237–50 | doi = 10.1007/s12013-011-9200-x | pmid = 21573992 }}</ref> The Pande lab is focusing their research on Alzheimer's with the goal of predicting the aggregate structure and how it develops for ] approaches as well as developing methods to stop the aggregation process.<ref name="diseases FAQ" /> In 2008 Folding@home simulated the dynamics of Aβ in atomic detail over timescales of the order of tens of seconds. This was significant as previous simulations were about six orders of magnitude shorter. Researchers used the resulting Markov state model to identify a ] that was a major source of molecular interactions within the structure.<ref name="MSM applications" |
Disabling Aβ aggregation using small molecules is regarded as a promising approach to the development of therapeutic drugs for treating Alzheimer's patients.<ref>{{cite journal | author = Aabgeena Naeem and Naveed Ahmad Fazili | title = Defective Protein Folding and Aggregation as the Basis of Neurodegenerative Diseases: The Darker Aspect of Proteins | format = review | journal = Cell Biochemistry and Biophysics | year = 2011 | volume = 61 | issue = 2 | pages = 237–50 | doi = 10.1007/s12013-011-9200-x | pmid = 21573992 }}</ref> The Pande lab is focusing their research on Alzheimer's with the goal of predicting the aggregate structure and how it develops for ] approaches as well as developing methods to stop the aggregation process.<ref name="diseases FAQ" /> In 2008 Folding@home simulated the dynamics of Aβ in atomic detail over timescales of the order of tens of seconds. This was significant as previous simulations were about six orders of magnitude shorter. Researchers used the resulting Markov state model to identify a ] that was a major source of molecular interactions within the structure.<ref name="MSM applications" /> This study helped prepare the Pande lab for future aggregation studies and for further research to find a small peptide which may stabilize the aggregation process.<ref name="Simulating oligomerization" /> In the same year, Folding@home found several small drug candidates which appear to inhibit the toxicity of Aβ.<ref>{{cite web | url = http://folding.typepad.com/news/2008/12/new-fah-results-on-possible-new-alzheimers-drug-presented.html | title = New FAH results on possible new Alzheimer's drug presented | author = Vijay Pande | work = Folding@home | publisher = ] | date = December 18, 2008 | accessdate = September 23, 2011 }}</ref> In 2010, in close cooperation with the Nanomedicine Center for Protein Folding, these drug leads went from the ] to testing on ].<ref name="diseases FAQ" /> In 2011, Folding@home completed simulations of several ]s of Aβ that appear to stabilize the aggregate formation, which could aid in the development of therapeutic drug approaches to the disease as well as greatly assisting with experimental ] studies of the oligomers.<ref name="Novick2011" /><ref>{{cite journal | author = Paul A. Novick, Dahabada H. Lopes, Kim M. Branson, Alexandra Esteras-Chopo, Isabella A. Graef, Gal Bitan, and Vijay S. Pande | title = Design of β-Amyloid Aggregation Inhibitors from a Predicted Structural Motif | journal = Journal of Medicinal Chemistry | year = 2012 | volume = 55 | issue = 7 | pages = 3002–10 | doi = 10.1021/jm201332p | pmid = 22420626 }}</ref> Later that year, Folding@home began simulations of various Aβ fragments in order to determine how various natural enzymes affect the structure and folding of Aβ.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=66&t=19201&p=191821 | title = New project p6871 | author = yslin (Pande lab member) | work = Folding@home | publisher = ] Group | date = July 22, 2011 | accessdate = March 17, 2012}}{{registration required }}</ref><ref>{{cite web | url = http://fah-web.stanford.edu/cgi-bin/fahproject.overusingIPswillbebanned?p=6871 | title = Project 6871 Description | author = Pande lab | work = Folding@home | publisher = ] | accessdate = September 27, 2011 }}</ref> | ||
| === Huntington's disease === | === Huntington's disease === | ||
| ] is a neurodegenerative ] that is also associated with protein misfolding and aggregation. ] of the ] amino acid at the ] of the ] cause aggregation, and although the behavior of the repeats is not completely understood, it does lead to the cognitive decline associated with the disease.<ref>{{cite journal | author = Walker FO | title = Huntington's disease | journal = Lancet | volume = 369 | issue = 9557 | page = 220 | year = 2007 | pmid = 17240289 | doi = 10.1016/S0140-6736(07)60111-1 | pages = 218–28 }}</ref> As with other aggregates, there is difficulty in experimentally determining its structure.<ref name="Huntingtin headpiece structure" |
] is a neurodegenerative ] that is also associated with protein misfolding and aggregation. ] of the ] amino acid at the ] of the ] cause aggregation, and although the behavior of the repeats is not completely understood, it does lead to the cognitive decline associated with the disease.<ref>{{cite journal | author = Walker FO | title = Huntington's disease | journal = Lancet | volume = 369 | issue = 9557 | page = 220 | year = 2007 | pmid = 17240289 | doi = 10.1016/S0140-6736(07)60111-1 | pages = 218–28 }}</ref> As with other aggregates, there is difficulty in experimentally determining its structure.<ref name="Huntingtin headpiece structure" /> Scientists are using Folding@home to study Huntingtin protein aggregate structure as well as to predict how the aggregate forms, assisting with ] approaches to stop the aggregate formation.<ref name="diseases FAQ" /> The N17 fragment of the Huntingtin protein accelerates this aggregation, and while there have been several proposed mechanisms, its exact role in this process remains largely unknown.<ref>{{cite journal | author = Susan W Liebman & Stephen C Meredith | title = Protein folding: Sticky N17 speeds huntingtin pile-up | journal = Nature — Chemical Biology | year = 2010 | volume = 6 | issue = 1 | pages = 7–8 | doi = 10.1038/nchembio.279 | pmid = 20016493 }}</ref> Folding@home has simulated this and other fragments in order to elucidate their roles in the disease.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=66&t=20765&p=207880 | title = Project 8021 released to beta | author = Diwakar Shukla (Pande lab member) | work = Folding@home | publisher = ] Group | date = February 10, 2012 | accessdate = March 17, 2012}}{{registration required }}</ref> Since 2008, its drug design approaches for Alzheimer's disease have been applied to Huntington's, and in 2010, Folding@home researcher Veena Thomas proposed a novel therapeutic strategy for Huntington's which may be funded by the ]. This strategy could be used to bring the results from Folding@home directly to a therapeutic drug.<ref name="diseases FAQ" /> | ||
| === Cancer === | === Cancer === | ||
| Line 65: | Line 65: | ||
| === Drug design === | === Drug design === | ||
| ]s function by ] to ] on target molecules and causing a certain desired change. Ideally, a drug should act very specifically and bind only to its target without interfering with other biological functions. However, it is difficult to precisely determine where and ] two molecules will bind. Due to limitations in computational power, current '']'' approaches usually have to trade speed for accuracy; ''e.g.'' use rapid ] methods instead of ] ]s. Folding@home's computational performance allows researchers to use both techniques, and evaluate their efficiency and reliability.<ref name="Press FAQ" /><ref name="New drug design methods" |
]s function by ] to ] on target molecules and causing a certain desired change. Ideally, a drug should act very specifically and bind only to its target without interfering with other biological functions. However, it is difficult to precisely determine where and ] two molecules will bind. Due to limitations in computational power, current '']'' approaches usually have to trade speed for accuracy; ''e.g.'' use rapid ] methods instead of ] ]s. Folding@home's computational performance allows researchers to use both techniques, and evaluate their efficiency and reliability.<ref name="Press FAQ" /><ref name="New drug design methods" /><ref name="POP comp" /> Computer-assisted ] has the potential to expedite and lower the costs of drug discovery.<ref name="Comp-aided drug design" /> In 2010, Folding@home used MSMs and free energy calculations to predict the ] of the ] protein to within 1.8 ] ] from the ] experimentally determined through ]. This may be important to future ] approaches, including for ].<ref name="MSM applications" /> Scientists have used Folding@home to research ] by studying ], an ], and ], a protein that can break down antibiotics like ]. They hope to be better able to design drugs to deactivate them.<ref>{{cite web | url = http://fah-web.stanford.edu/cgi-bin/fahproject.overusingIPswillbebanned?p=10721 | title = Project 10721 Description | author = Pande lab | work = Folding@home | publisher = ] | accessdate = September 27, 2011 }}</ref><ref name="drug target search" /> | ||
| Chemical activity occurs along a protein's ]. Traditional drug design approaches involve tightly binding to this site and blocking its activity, under the assumption that the target protein exists in a single rigid structure. However, this approach only works for approximately 15% of all proteins. Proteins contain ]s which, when bound to by small molecules, can alter a protein's conformation and ultimately affect the protein's activity. These sites are attractive drug targets, but locating them is very computationally expensive. In 2012, Folding@home and MSMs were used to identify allosteric site in three medically relevant proteins: ], ], and ].<ref name="drug target search" /><ref>{{cite journal | title = Equilibrium fluctuations of a single folded protein reveal a multitude of potential cryptic allosteric sites | author = Gregory R. Bowman and Phillip L. Geissler | journal = ] | year = 2012 | month = July | volume = 109 | issue = 29 | page = 11681 | doi = 10.1073/pnas.1209309109 | bibcode = 2012PNAS..10911681B }}</ref> | Chemical activity occurs along a protein's ]. Traditional drug design approaches involve tightly binding to this site and blocking its activity, under the assumption that the target protein exists in a single rigid structure. However, this approach only works for approximately 15% of all proteins. Proteins contain ]s which, when bound to by small molecules, can alter a protein's conformation and ultimately affect the protein's activity. These sites are attractive drug targets, but locating them is very computationally expensive. In 2012, Folding@home and MSMs were used to identify allosteric site in three medically relevant proteins: ], ], and ].<ref name="drug target search" /><ref>{{cite journal | title = Equilibrium fluctuations of a single folded protein reveal a multitude of potential cryptic allosteric sites | author = Gregory R. Bowman and Phillip L. Geissler | journal = ] | year = 2012 | month = July | volume = 109 | issue = 29 | page = 11681 | doi = 10.1073/pnas.1209309109 | bibcode = 2012PNAS..10911681B }}</ref> | ||
| Line 74: | Line 74: | ||
| ] client was released.]] | ] client was released.]] | ||
| In addition to reporting active processors, Folding@home also determines its computing performance as measured in ] based on the actual execution time of its calculations. Originally this was reported as native FLOPS, that is, the raw performance from each given type of processing hardware.<ref name="FLOPS FAQ" |
In addition to reporting active processors, Folding@home also determines its computing performance as measured in ] based on the actual execution time of its calculations. Originally this was reported as native FLOPS, that is, the raw performance from each given type of processing hardware.<ref name="FLOPS FAQ" /> In March 2009 Folding@home began reporting the performance in both native and x86 FLOPS:<ref>{{cite web | url = http://folding.typepad.com/news/2009/03/flops.html | title = FLOPS | author = Vijay Pande | work = Folding@home | publisher = ] | date = March 18, 2009 | accessdate = October 11, 2011 }}</ref> the latter being an estimation of how many FLOPS the calculation would take on the standard ] architecture, which is commonly used as a performance reference. Specialized hardware such as GPUs can efficiently perform certain complex functions in a single FLOP which would otherwise require multiple FLOPS on the x86 architecture. This x86 measurement attempts to even out these hardware differences.<ref name="FLOPS FAQ" /> Despite using conservative conversions, for the GPU and PS3 clients x86 FLOPS are consistently much greater than their native FLOPS and comprise a large majority of Folding@home's FLOP performance.<ref name="osstats" /><ref name="PS3 FAQ" /> | ||
| In 2007 ] recognized Folding@home as the most powerful distributed computing network in the world.<ref>{{cite web | url = http://www.guinnessworldrecords.com/world-records/5000/most-powerful-distributed-computing-network | title = Most powerful distributed computing network | work = Guinnessworldrecords.com | publisher = ] | date = September 16, 2007 | accessdate = April 6, 2012 }}</ref> As of September 13, 2012, the project has 217,215 active ]s, 19,623 active ]s, and 16,252 active ], for a total of 3.654 native ] (5.354 ] petaFLOPS).<ref name="osstats" |
In 2007 ] recognized Folding@home as the most powerful distributed computing network in the world.<ref>{{cite web | url = http://www.guinnessworldrecords.com/world-records/5000/most-powerful-distributed-computing-network | title = Most powerful distributed computing network | work = Guinnessworldrecords.com | publisher = ] | date = September 16, 2007 | accessdate = April 6, 2012 }}</ref> As of September 13, 2012, the project has 217,215 active ]s, 19,623 active ]s, and 16,252 active ], for a total of 3.654 native ] (5.354 ] petaFLOPS).<ref name="osstats" /> At the same time, the combined efforts of all distributed computing projects under BOINC totals 6.174 petaFLOPS from 447,720 active hosts.<ref>{{cite web | url = http://boincstats.com/en/stats/-1/project/detail | title = BOINC Combined Credit Overview | work = BOINCstats.com | publisher = BOINC Stats | accessdate = June 12, 2012 }}</ref> Using the Markov state model approach, Folding@home achieves ] across its user base and gains a near-linear speedup for every additional processor.<ref name="Everything about MSMs" /><ref name="taming folding complexity" /> This large and powerful network allows Folding@home to do work not possible any other way.<ref name="biologists think bigger" /> | ||
| Active participation in Folding@home has grown steadily since its launch.<ref>{{cite web | url = http://folding.typepad.com/news/2007/10/fun-fact-fah-gr.html | title = Fun fact: FAH growth over time | author = Vijay Pande | work = Folding@home | publisher = ] | date = October 21, 2007 | accessdate = October 21, 2011 }}</ref><ref>{{cite web | url = http://www.stanford.edu/group/pandegroup/images/ActiveCPUs2010.png | title = Active CPUs | format = Image | author = Pande lab | work = Folding@home | publisher = ] | accessdate = August 30, 2011 }}</ref> In March 2002 ] co-founder ] launched ] as add-on for the ].<ref>{{cite news | url = http://news.cnet.com/2100-1001-867091.html | title = Google takes on supercomputing | date = March 22, 2002 | publisher = CNet News | first = Stephen | last = Shankland }}</ref> Although limited in functionality and scope, it increased participation in Folding@home from 10,000 up to about 30,000 active CPUs.<ref name="Biotech 27" |
Active participation in Folding@home has grown steadily since its launch.<ref>{{cite web | url = http://folding.typepad.com/news/2007/10/fun-fact-fah-gr.html | title = Fun fact: FAH growth over time | author = Vijay Pande | work = Folding@home | publisher = ] | date = October 21, 2007 | accessdate = October 21, 2011 }}</ref><ref>{{cite web | url = http://www.stanford.edu/group/pandegroup/images/ActiveCPUs2010.png | title = Active CPUs | format = Image | author = Pande lab | work = Folding@home | publisher = ] | accessdate = August 30, 2011 }}</ref> In March 2002 ] co-founder ] launched ] as add-on for the ].<ref>{{cite news | url = http://news.cnet.com/2100-1001-867091.html | title = Google takes on supercomputing | date = March 22, 2002 | publisher = CNet News | first = Stephen | last = Shankland }}</ref> Although limited in functionality and scope, it increased participation in Folding@home from 10,000 up to about 30,000 active CPUs.<ref name="Biotech 27" /> The program ended in October 2005 in favor of the official Folding@home clients, and is no longer available for the Toolbar.<ref>{{cite news | url = http://toolbar.google.com/dc/offerdc.html/ | archiveurl = http://web.archive.org/web/20080611153319/http://toolbar.google.com/dc/offerdc.html/ | archivedate = June 11, 2008 | title = Your computer's idle time is too precious to waste | author = Google | year = 2007 | accessdate = August 31, 2012 }}</ref> Folding@home also gained participants from ], another distributed computing project from the Pande lab and a sister project to Folding@home. The goal of Genome@home was ] and associated applications. Following its official conclusion in March 2004, users were asked to donate computing power to Folding@home instead.<ref>{{cite news | url = http://www.stanford.edu/group/pandegroup/genome/new.html | title = Genome@home Updates | author = Vijay Pande, Stefan Larson | at = April 15, 2004 Update | date = March 4, 2002 | accessdate = March 17, 2012 }}</ref> | ||
| === Performance === | === Performance === | ||
| {| class="wikitable" style="float:right; margin-left: |
{| class="wikitable" style="float: right; margin-right: 0; margin-left: 1em;" | ||
| |- | |- | ||
| ! Date | ! Date | ||
| Line 100: | Line 100: | ||
| |- | |- | ||
| | style="text-align: center;" | November 2008 | | style="text-align: center;" | November 2008 | ||
| | style="text-align: center;" | 4,021 TFLOPS<ref name="past 4 petaFLOPS" |
| style="text-align: center;" | 4,021 TFLOPS<ref name="past 4 petaFLOPS" /> | ||
| | style="text-align: center;" | 1,105 TFLOP Roadrunner<ref>{{cite web | url = http://www.top500.org/list/2008/11/100 | title = TOP500 List — November 2008 | work = top500.org | publisher = ] | date = November 2008 | accessdate = August 12, 2012 }}</ref> | | style="text-align: center;" | 1,105 TFLOP Roadrunner<ref>{{cite web | url = http://www.top500.org/list/2008/11/100 | title = TOP500 List — November 2008 | work = top500.org | publisher = ] | date = November 2008 | accessdate = August 12, 2012 }}</ref> | ||
| |- | |- | ||
| | style="text-align: center;" | June 2009 | | style="text-align: center;" | June 2009 | ||
| | style="text-align: center;" | 4,668 native, 8,418 x86 TFLOPS<ref>{{cite web | url = http://translate.google.com/translate?hl=en&sl=zh-CN&u=http://www.equn.com/forum/thread-18638-32-5.html | title = Google Translate |
| style="text-align: center;" | 4,668 native, 8,418 x86 TFLOPS<ref>{{cite web | url = http://translate.google.com/translate?hl=en&sl=zh-CN&u=http://www.equn.com/forum/thread-18638-32-5.html | title = Google Translate – Distributed Computing Forum in Chinese |publisher=Google | date = June 1, 2009 | accessdate = August 24, 2012 }}</ref> | ||
| | style="text-align: center;" | 1,105 TFLOP Roadrunner<ref>{{cite web | url = http://www.top500.org/list/2009/06/100 | title = TOP500 List — June 2009 | work = top500.org | publisher = ] | date = June 2009 | accessdate = August 12, 2012 }}</ref> | | style="text-align: center;" | 1,105 TFLOP Roadrunner<ref>{{cite web | url = http://www.top500.org/list/2009/06/100 | title = TOP500 List — June 2009 | work = top500.org | publisher = ] | date = June 2009 | accessdate = August 12, 2012 }}</ref> | ||
| |- | |- | ||
| Line 112: | Line 112: | ||
| |- | |- | ||
| | style="text-align: center;" | June 2010 | | style="text-align: center;" | June 2010 | ||
| | style="text-align: center;" | 3,249 native, 5,674 x86 TFLOPS<ref name="FAH Stats doc" |
| style="text-align: center;" | 3,249 native, 5,674 x86 TFLOPS<ref name="FAH Stats doc" /> | ||
| | style="text-align: center;" | 1,759 TFLOP Jaguar<ref>{{cite web | url = http://www.top500.org/list/2010/06/100 | title = TOP500 List — June 2010 | work = top500.org | publisher = ] | date = June 2010 | accessdate = August 12, 2012 }}</ref> | | style="text-align: center;" | 1,759 TFLOP Jaguar<ref>{{cite web | url = http://www.top500.org/list/2010/06/100 | title = TOP500 List — June 2010 | work = top500.org | publisher = ] | date = June 2010 | accessdate = August 12, 2012 }}</ref> | ||
| |- | |- | ||
| Line 120: | Line 120: | ||
| |- | |- | ||
| | style="text-align: center;" | June 2011 | | style="text-align: center;" | June 2011 | ||
| | style="text-align: center;" | 5,706 native, 9,359 x86 TFLOPS<ref>{{cite web | url = http://www.itocp.com/bbs/thread-100756-1-1.html | title = 星空下闪耀的双子星—影驰GTX560黑将试用Ⅴ. 通用运算及总结 |
| style="text-align: center;" | 5,706 native, 9,359 x86 TFLOPS<ref>{{cite web | url = http://www.itocp.com/bbs/thread-100756-1-1.html | title = 星空下闪耀的双子星—影驰GTX560黑将试用Ⅴ. 通用运算及总结 – 玩家堂官方硬件团购及活动区 – 玩家堂论坛-硬件爱好者和电脑玩家的天堂 | author = flight8848 | language = Chinese | publisher = itocp.com | date = June 9, 2011 | accessdate = September 16, 2012 }}</ref> | ||
| | style="text-align: center;" | 8,162 TFLOP ]<ref>{{cite web | url = http://www.top500.org/list/2011/06/100 | title = TOP500 List — June 2011 | work = top500.org | publisher = ] | date = June 2011 | accessdate = August 12, 2012 }}</ref> | | style="text-align: center;" | 8,162 TFLOP ]<ref>{{cite web | url = http://www.top500.org/list/2011/06/100 | title = TOP500 List — June 2011 | work = top500.org | publisher = ] | date = June 2011 | accessdate = August 12, 2012 }}</ref> | ||
| |- | |- | ||
| | style="text-align: center;" | November 2011 | | style="text-align: center;" | November 2011 | ||
| | style="text-align: center;" | 6,012 native, 7,942 x86 TFLOPS<ref name="6 petaFLOPS" |
| style="text-align: center;" | 6,012 native, 7,942 x86 TFLOPS<ref name="6 petaFLOPS" /> | ||
| | style="text-align: center;" | 10,510 TFLOP K computer<ref>{{cite web | url = http://www.top500.org/list/2011/11/100 | title = TOP500 List — November 2011 | work = top500.org | publisher = ] | date = November 2011 | accessdate = August 12, 2012 }}</ref> | | style="text-align: center;" | 10,510 TFLOP K computer<ref>{{cite web | url = http://www.top500.org/list/2011/11/100 | title = TOP500 List — November 2011 | work = top500.org | publisher = ] | date = November 2011 | accessdate = August 12, 2012 }}</ref> | ||
| |- | |- | ||
| Line 135: | Line 135: | ||
| == Points == | == Points == | ||
| Similarly to other distributed computing projects, Folding@home quantitatively assesses user computing contributions to the project through a credit system.<ref name="Points FAQ" |
Similarly to other distributed computing projects, Folding@home quantitatively assesses user computing contributions to the project through a credit system.<ref name="Points FAQ" /> All units from a given protein project have uniform base credit, which is determined by benchmarking one or more Work Units from that project on an official reference machine before the project is released.<ref name="PointsNew FAQ" /> Each user receives these base points for completing every Work Unit, though through the use of a passkey they can receive additional bonus points for reliably and rapidly completing units which are more computationally demanding or have a greater scientific priority.<ref>{{cite web | url = http://folding.stanford.edu/English/FAQ-passkey | title = Folding@home Passkey FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = July 23, 2012 | accessdate = August 20, 2012 }}</ref><ref name="SMP2 release" /> Users may also receive credit for their work by clients on multiple machines.<ref name="Main FAQ" /> This generates a fair system of equal pay for equal work, and attempts to align credit with the value of the scientific results.<ref name="Points FAQ" /><ref name="PointsNew FAQ" /> | ||
| Users can register their contributions under a team, which combine the points of all their members. A user can start their own team, or they can join an existing team.<ref name="FAH homepage" |
Users can register their contributions under a team, which combine the points of all their members. A user can start their own team, or they can join an existing team.<ref name="FAH homepage" /> In some cases, a team may have their own community-driven sources of help or recruitment such as an ].<ref>{{cite web | url = http://forums.extremeoverclocking.com/forumdisplay.php?f=45 | title = Official Extreme Overclocking Folding@home Team Forum | work = forums.extremeoverclocking.com | publisher = Extreme Overclocking | accessdate = April 5, 2012 }}</ref> The points can foster friendly competition between individuals and teams to compute the most for the project, which can benefit the folding community and accelerate scientific research.<ref name="Points FAQ" /><ref name="lessons from 8 years" /><ref>{{cite web | url = http://www.maximumpc.com/article/news/help_maximum_pcs_folding_team_win_next_chimp_challenge | title = Help Maximum PC's Folding Team Win the Next Chimp Challenge! | author = Norman Chan | work = Maximumpc.com | publisher = Future US, Inc. | date = April 6, 2009 | accessdate = September 6, 2011 }}</ref> Individual and team statistics are posted on the Folding@home website.<ref name="Points FAQ" /> | ||
| == Software == | == Software == | ||
| Line 149: | Line 149: | ||
| === Cores === | === Cores === | ||
| {{main|Folding@home cores}} | {{main|Folding@home cores}} | ||
| Specialized molecular dynamics programs, referred to as "FahCores" and often abbreviated "cores", perform the calculations on the Work Unit behind the scenes. A large majority of Folding@home's cores are based on ],<ref name="lessons from 8 years" /> one of the fastest and most popular molecular dynamics software packages available, which largely consists of manually optimized ] and hardware optimizations.<ref>{{cite journal | author = Carsten Kutzner, David Van Der Spoel, Martin Fechner, Erik Lindahl, Udo W. Schmitt, Bert L. De Groot, and Helmut Grubmüller | title = Speeding up parallel GROMACS on high-latency networks | journal = Journal of Computational Chemistry | year = 2007 | volume = 28 | issue = 12 | pages = 2075–2084 | doi = 10.1002/jcc.20703 | pmid = 17405124 }}</ref><ref>{{cite journal | author = Berk Hess, Carsten Kutzner, David van der Spoel, and Erik Lindahl | title = GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation | journal = Journal of Chemical Theory and Computation | year = 2008 | volume = 4 | issue = 3 | pages = 435–447 | doi = 10.1021/ct700301q }}</ref> Although GROMACS is ] and there is a cooperative effort between the Pande lab and GROMACS developers, Folding@home uses a ] license for data validity reasons.<ref>{{cite web | url = http://folding.stanford.edu/English/FAQ-gromacs | title = Folding@home Gromacs FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 19, 2012 | accessdate = August 20, 2012 }}</ref> Less active cores include ProtoMol and SHARPEN. Folding@home has used ], ], ], and ], but these have since been retired and are no longer in active service.<ref name="Open Source FAQ" |
Specialized molecular dynamics programs, referred to as "FahCores" and often abbreviated "cores", perform the calculations on the Work Unit behind the scenes. A large majority of Folding@home's cores are based on ],<ref name="lessons from 8 years" /> one of the fastest and most popular molecular dynamics software packages available, which largely consists of manually optimized ] and hardware optimizations.<ref>{{cite journal | author = Carsten Kutzner, David Van Der Spoel, Martin Fechner, Erik Lindahl, Udo W. Schmitt, Bert L. De Groot, and Helmut Grubmüller | title = Speeding up parallel GROMACS on high-latency networks | journal = Journal of Computational Chemistry | year = 2007 | volume = 28 | issue = 12 | pages = 2075–2084 | doi = 10.1002/jcc.20703 | pmid = 17405124 }}</ref><ref>{{cite journal | author = Berk Hess, Carsten Kutzner, David van der Spoel, and Erik Lindahl | title = GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation | journal = Journal of Chemical Theory and Computation | year = 2008 | volume = 4 | issue = 3 | pages = 435–447 | doi = 10.1021/ct700301q }}</ref> Although GROMACS is ] and there is a cooperative effort between the Pande lab and GROMACS developers, Folding@home uses a ] license for data validity reasons.<ref>{{cite web | url = http://folding.stanford.edu/English/FAQ-gromacs | title = Folding@home Gromacs FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 19, 2012 | accessdate = August 20, 2012 }}</ref> Less active cores include ProtoMol and SHARPEN. Folding@home has used ], ], ], and ], but these have since been retired and are no longer in active service.<ref name="Open Source FAQ" /><ref>{{cite web | url = http://folding.stanford.edu/English/FAQ | title = Folding@home Frequently Asked Questions (FAQ) Index | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 7, 2012 | accessdate = August 20, 2012 }}</ref><ref>{{cite web | url = http://folding.typepad.com/news/2009/09/update-on-new-fah-cores-and-clients.html | title = Update on new FAH cores and clients | author = Vijay Pande | work = Folding@home | publisher = ] | date = September 25, 2009 | accessdate = February 24, 2012 }}</ref> Some of these cores perform ] calculations in which the surrounding ] (usually water) is modeled atom-by-atom; while others perform ] methods, where the solvent is treated as a mathematical continuum.<ref name="Acc. MD on GPU" /><ref>{{cite web | url = http://folding.stanford.edu/English/FAQ-Petaflop | title = Folding@home Petaflop Initiative | author = Pande lab | work = Folding@home | publisher = ] | date = August 19, 2012 | accessdate = August 20, 2012 }}</ref> The core is separate from the client to enable the scientific methods to be updated automatically without requiring a client update. The cores periodically create calculation ] so that if they are interrupted they can resume work from that point upon startup.<ref name="lessons from 8 years" /> | ||
| === Client === | === Client === | ||
| Folding@home participants install a ] ] on their ] or on the ] gaming console. The user interacts with the client, which manages the other software components behind the scenes. Through the client, the user may pause the folding process, open an event log, check the work progress, or view personal statistics.<ref name="Uni Guide" |
Folding@home participants install a ] ] on their ] or on the ] gaming console. The user interacts with the client, which manages the other software components behind the scenes. Through the client, the user may pause the folding process, open an event log, check the work progress, or view personal statistics.<ref name="Uni Guide" /> The computer clients run continuously in the ] at an extremely low priority, utilizing otherwise unused processing power so that normal computer usage is unaffected.<ref name="FAH homepage" /><ref name="Main FAQ" /> The maximum CPU utilization can also be adjusted through client settings.<ref name="Uni Guide" /><ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=50&t=15863#p157125 | title = Re: Can Folding@home damage any part of my PC? | author = PantherX | work = Folding@home | publisher = ] Group | date = September 2, 2010 | accessdate = February 25, 2012 }}</ref> The client connects to a Folding@home ] and retrieves a Work Unit and may also download the appropriate core for the client's settings, operating system, and the underlying hardware architecture. After processing, the Work Unit is returned to the Folding@home servers. Computer clients tailor to ] and ]s systems, as well as ]s. While these latter clients use significantly more resources, the diversity and power of each ] provides Folding@home with the ability to efficiently complete many different types of simulations in a timely manner, (in a few weeks or months rather than years) which is of significant scientific value. Together, these clients allow researchers to study biomedical questions previously considered impossible to tackle computationally.<ref name="Press FAQ" /><ref name="SMP FAQ" /> | ||
| Folding@home software developers put significant work goes into minimizing security issues. For example, clients can be downloaded only from the official Folding@home website or its commercial partners.<ref name="Main FAQ" /><ref name="license" |
Folding@home software developers put significant work goes into minimizing security issues. For example, clients can be downloaded only from the official Folding@home website or its commercial partners.<ref name="Main FAQ" /><ref name="license" /> Folding@home's ] forbids public access to the client ] for security and scientific integrity reasons.<ref name="license" /><ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=24&t=3600 | title = Folding@home's End User License Agreement (EULA) | author = Vijay Pande | work = Folding@home | date = June 28, 2008 | accessdate = May 15, 2012 }}</ref> Each client will upload and download data only from Stanford's Folding@home data servers (over ] 8080, with 80 as an alternative) using 2048-bit ] for verification and will only interact with Folding@home computer files.<ref name="Main FAQ" /><ref name="uninstall" /> Thus from a security standpoint it behaves in a similar fashion to a ], but is even more secure.<ref name="Biotech 27" /><ref name="uninstall" /> | ||
| Folding@home's first client was a ], which would run Folding@home while the computer was not otherwise in use.<ref>{{cite journal | author = M. R. Shirts and V. S. Pande. | title = Screen Savers of the World, Unite! | journal = Science | year = 2000 | volume = 290 | issue = 5498 | pages = 1903–1904 | doi = 10.1126/science.290.5498.1903 | pmid = 17742054 }}</ref> In 2004 the Pande lab collaborated with ] to test a supplemental client on the open-source BOINC framework.<ref>{{cite web | url = http://www.boarddigger.com/forum/gxbovGYpO1F | title = Folding@home client for BOINC in beta "soon" | author = Rattledagger, Vijay Pande | work = Boarddigger.com | publisher = Anandtech.com | date = April 1, 2005 | accessdate = April 5, 2012 }}</ref> This client was released to closed beta in April 2005; however, the approach became unworkable and was abandoned in June 2006.<ref name="highper FAQ" |
Folding@home's first client was a ], which would run Folding@home while the computer was not otherwise in use.<ref>{{cite journal | author = M. R. Shirts and V. S. Pande. | title = Screen Savers of the World, Unite! | journal = Science | year = 2000 | volume = 290 | issue = 5498 | pages = 1903–1904 | doi = 10.1126/science.290.5498.1903 | pmid = 17742054 }}</ref> In 2004 the Pande lab collaborated with ] to test a supplemental client on the open-source BOINC framework.<ref>{{cite web | url = http://www.boarddigger.com/forum/gxbovGYpO1F | title = Folding@home client for BOINC in beta "soon" | author = Rattledagger, Vijay Pande | work = Boarddigger.com | publisher = Anandtech.com | date = April 1, 2005 | accessdate = April 5, 2012 }}</ref> This client was released to closed beta in April 2005; however, the approach became unworkable and was abandoned in June 2006.<ref name="highper FAQ" /> BOINC's fixed architecture limits the types of project it can accommodate and thus was not appropriate for Folding@home.<ref name="lessons from 8 years" /> | ||
| ==== Graphics processing units ==== | ==== Graphics processing units ==== | ||
| The specialized hardware of ]s is designed to accelerate rendering of 3D graphics applications such as video games and can significantly outperform CPUs for certain types of calculations. Although limited in generality, this makes GPUs one of the most powerful and rapidly growing computational platforms. As such, ] is the pursuit of many scientists and researchers. However, GPU hardware is difficult to utilize for non-graphics tasks and usually requires significant algorithm restructuring and an advanced understanding of the underlying architecture.<ref>{{cite journal | author = John D. Owens, David Luebke, Naga Govindaraju, Mark Harris, Jens Krüger, Aaron Lefohn, Timothy J. Purcell | title = A Survey of General-Purpose Computation on Graphics Hardware | journal = Computer Graphics Forum | year = 2007 | volume = 26 | issue = 1 | pages = 80–113 | doi = 10.1111/j.1467-8659.2007.01012.x }}</ref> Such customization is challenging, especially to researchers with limited software development resources. Folding@home uses the ] ] ], which uses two ] levels to interface molecular simulation software to an underlying hardware architecture. With the addition of hardware optimizations, OpenMM-based GPU simulations do not require significant modification but achieve performance nearly equal to hand-tuned GPU code, and greatly outperform CPU implementations.<ref name="Acc. MD on GPU" |
The specialized hardware of ]s is designed to accelerate rendering of 3D graphics applications such as video games and can significantly outperform CPUs for certain types of calculations. Although limited in generality, this makes GPUs one of the most powerful and rapidly growing computational platforms. As such, ] is the pursuit of many scientists and researchers. However, GPU hardware is difficult to utilize for non-graphics tasks and usually requires significant algorithm restructuring and an advanced understanding of the underlying architecture.<ref>{{cite journal | author = John D. Owens, David Luebke, Naga Govindaraju, Mark Harris, Jens Krüger, Aaron Lefohn, Timothy J. Purcell | title = A Survey of General-Purpose Computation on Graphics Hardware | journal = Computer Graphics Forum | year = 2007 | volume = 26 | issue = 1 | pages = 80–113 | doi = 10.1111/j.1467-8659.2007.01012.x }}</ref> Such customization is challenging, especially to researchers with limited software development resources. Folding@home uses the ] ] ], which uses two ] levels to interface molecular simulation software to an underlying hardware architecture. With the addition of hardware optimizations, OpenMM-based GPU simulations do not require significant modification but achieve performance nearly equal to hand-tuned GPU code, and greatly outperform CPU implementations.<ref name="Acc. MD on GPU" /><ref>{{cite journal | author = P. Eastman and V. S. Pande | title = OpenMM: A Hardware-Independent Framework for Molecular Simulations | journal = Computing in Science and Engineering | year = 2010 | volume = 12 | issue = 4 | pages = 34–39 | doi = 10.1109/MCSE.2010.27 | issn = 1521-9615 }}</ref> | ||
| Prior to 2010 the computational reliability of GPGPU consumer-grade hardware had remained largely unknown, and circumstantial evidence related to the lack of built-in ] in GPU memory raised reliability concerns. In the first large-scale test of GPU scientific accuracy, a 2010 study of over 20,000 hosts on the Folding@home network detected ]s in the memory subsystems of two-thirds of the tested GPUs. These errors strongly correlated to board architecture, though the study concluded that reliable GPU computing was very feasible as long as attention is paid to the hardware characteristics, such as through the use of software-side error detection.<ref>{{cite journal | author = I. Haque and V. S. Pande | title = Hard Data on Soft Errors: A Large-Scale Assessment of Real-World Error Rates in GPGPU | journal = 2010 10th IEEE/ACM International Conference on Cluster, Cloud and Grid Computing (CCGrid) | year = 2010 | pages = 691–696 | doi = 10.1109/CCGRID.2010.84 | isbn = 978-1-4244-6987-1 }}</ref> | Prior to 2010 the computational reliability of GPGPU consumer-grade hardware had remained largely unknown, and circumstantial evidence related to the lack of built-in ] in GPU memory raised reliability concerns. In the first large-scale test of GPU scientific accuracy, a 2010 study of over 20,000 hosts on the Folding@home network detected ]s in the memory subsystems of two-thirds of the tested GPUs. These errors strongly correlated to board architecture, though the study concluded that reliable GPU computing was very feasible as long as attention is paid to the hardware characteristics, such as through the use of software-side error detection.<ref>{{cite journal | author = I. Haque and V. S. Pande | title = Hard Data on Soft Errors: A Large-Scale Assessment of Real-World Error Rates in GPGPU | journal = 2010 10th IEEE/ACM International Conference on Cluster, Cloud and Grid Computing (CCGrid) | year = 2010 | pages = 691–696 | doi = 10.1109/CCGRID.2010.84 | isbn = 978-1-4244-6987-1 }}</ref> | ||
| The first generation of Folding@home's ] GPU client (GPU1) was released to the public on October 2, 2006,<ref name="highper FAQ" /> delivering a 20-30X speedup for certain calculations over its CPU-based ] counterparts.<ref name="ATI FAQ" |
The first generation of Folding@home's ] GPU client (GPU1) was released to the public on October 2, 2006,<ref name="highper FAQ" /> delivering a 20-30X speedup for certain calculations over its CPU-based ] counterparts.<ref name="ATI FAQ" /> It was the first time GPUs had been used for either distributed computing or major molecular dynamics calculations.<ref>{{cite web | url = http://folding.typepad.com/news/2008/05/gpu-news-gpu1-g.html | title = GPU news (about GPU1, GPU2, & NVIDIA support) | author = Vijay Pande | work = Folding@home | publisher = ] | date = May 23, 2008 | accessdate = September 8, 2011 }}</ref><ref>{{cite book | author = Travis Desell, Anthony Waters, Malik Magdon-Ismail, Boleslaw K. Szymanski, Carlos A. Varela, Matthew Newby, Heidi Newberg, Andreas Przystawik, and David Anderson | chapter = Accelerating the MilkyWay@Home volunteer computing project with GPUs | title = 8th International Conference on Parallel Processing and Applied Mathematics (PPAM 2009) Part I | year = 2009 | pages = 276–288 | url = http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.158.7614 | isbn = 978-3-642-14389-2 }}</ref> GPU1 gave researchers significant knowledge and experience with the development of ] software, but in response to scientific inaccuracies with ], on April 10, 2008 it was succeeded by GPU2, the second generation of the client.<ref name="ATI FAQ" /><ref>{{cite web | url = http://folding.typepad.com/news/2008/04/gpu2-open-beta.html | title = GPU2 open beta | author = Vijay Pande | work = Folding@home | publisher = ] | date = April 10, 2008 | accessdate = September 7, 2011 }}</ref> Following its introduction, GPU1 was officially retired on June 6.<ref name="ATI FAQ" /> Compared to GPU1, GPU2 was more scientifically reliable and productive, ran on both ] and ]-enabled ] GPUs, and supported more advanced algorithms, larger proteins, and real-time visualization of the protein simulation.<ref>{{cite web | url = http://folding.typepad.com/news/2008/04/updates-to-the.html | title = Updates to the Download page/GPU2 goes live | author = Vijay Pande | work = Folding@home | publisher = ] | date = April 15, 2008 | accessdate = September 7, 2011 }}</ref><ref>{{cite web | url = http://folding.typepad.com/news/2008/04/gpu2-open-bet-1.html | title = GPU2 open beta going well | author = Vijay Pande | work = Folding@home | publisher = ] | date = April 11, 2008 | accessdate = September 7, 2011 }}</ref> Following this, the third generation of Folding@home's GPU client (GPU3) was released on May 25, 2010. While ] to GPU2, GPU3 was comparatively more stable and efficient, had greater flexibility in its scientific capabilities,<ref name="GPU3 prep" /> and used OpenMM on top of an ] framework.<ref name="GPU3 prep" /><ref>{{cite web | url = http://folding.typepad.com/news/2010/05/open-beta-release-of-the-gpu3-clientcore.html | title = Folding@home: Open beta release of the GPU3 client/core | author = Vijay Pande | work = Folding@home | publisher = ] | date = May 25, 2010 | accessdate = September 7, 2011 }}</ref> Although the GPU clients do not natively support the ] operating system, users with Nvidia graphics cards can run them under the ] software.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=67&t=19795&start=45#p197198 | title = Re: FAHClient V7.1.38 released (4th Open-Beta) | author = Joseph Coffland (Pande lab member) | work = Folding@home | publisher = ] Group | date = October 13, 2011 | accessdate = October 15, 2011 }}</ref><ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=54&t=6793 | title = NVIDIA GPU3 Linux/Wine Headless Install Guide | work = Folding@home | publisher = ] Group | date = November 8, 2008 | accessdate = September 5, 2011 }}</ref> GPUs remain Folding@home's most powerful platform in terms of ]; as of September 2012 GPU clients account for 76% of the entire project's x86 FLOP throughput.<ref name="osstats" /> | ||
| ==== PlayStation 3 ==== | ==== PlayStation 3 ==== | ||
| Line 169: | Line 169: | ||
| ] | ] | ||
| Folding@home can also take advantage of the computing power of PlayStation 3's. At the time of its inception and for certain calculations, its main ] ] delivered a 20x speed increase over PCs, processing power which could not be found on other systems such as the ].<ref name="Press FAQ" /><ref name="Biotech 27" /> The PS3's high speed and efficiency introduced other opportunities for ] optimizations, and significantly changed the tradeoff between computational efficiency and overall accuracy, allowing for the utilization of more complex molecular models at little additional computational cost.<ref name="Acc MD on PS3 Cell" |
Folding@home can also take advantage of the computing power of PlayStation 3's. At the time of its inception and for certain calculations, its main ] ] delivered a 20x speed increase over PCs, processing power which could not be found on other systems such as the ].<ref name="Press FAQ" /><ref name="Biotech 27" /> The PS3's high speed and efficiency introduced other opportunities for ] optimizations, and significantly changed the tradeoff between computational efficiency and overall accuracy, allowing for the utilization of more complex molecular models at little additional computational cost.<ref name="Acc MD on PS3 Cell" /> This allowed Folding@home to run biomedical calculations that would otherwise be computationally infeasible.<ref name="cnn ps3" /> | ||
| The PS3 client was developed in a collaborative effort between ] and the Pande lab and was first released as a standalone client on March 23, 2007.<ref name="Press FAQ" /><ref>{{cite web | url = http://www.mb.com.ph/node/11811 | title = The Home Cure: PlayStation 3 to Help Study Causes of Cancer | author = Jerry Liao | work = mb.com | publisher = Manila Bulletin Publishing Corporation | date = March 23, 2007 | accessdate = April 5, 2012 }}</ref> Its release made Folding@home the first distributed computing project to utilize PS3s.<ref>{{cite web | url = http://old.post-gazette.com/pg/07085/772011-96.stm | title = Week in video-game news: 'God of War II' storms the PS2; a PS3 research project | author = Lou Kesten, ] | work = Post-Gazette.com | publisher = ] | date = March 26, 2007 | accessdate = April 5, 2012 }}</ref> On September 18 of the following year, the PS3 client became a channel of ] on its launch.<ref>{{cite web | url = http://gizmodo.com/5051558/ps3-news-service-life-with-playstation-now-up-for-download | title = PS3 News Service, Life With Playstation, Now Up For Download | author = Elaine Chow | work = Gizmodo.com | publisher = ] | date = September 18, 2008 | accessdate = April 5, 2012 }}</ref><ref>{{cite web | url = http://folding.typepad.com/news/2008/09/life-with-playstation.html | title = Life with Playstation |
The PS3 client was developed in a collaborative effort between ] and the Pande lab and was first released as a standalone client on March 23, 2007.<ref name="Press FAQ" /><ref>{{cite web | url = http://www.mb.com.ph/node/11811 | title = The Home Cure: PlayStation 3 to Help Study Causes of Cancer | author = Jerry Liao | work = mb.com | publisher = Manila Bulletin Publishing Corporation | date = March 23, 2007 | accessdate = April 5, 2012 }}</ref> Its release made Folding@home the first distributed computing project to utilize PS3s.<ref>{{cite web | url = http://old.post-gazette.com/pg/07085/772011-96.stm | title = Week in video-game news: 'God of War II' storms the PS2; a PS3 research project | author = Lou Kesten, ] | work = Post-Gazette.com | publisher = ] | date = March 26, 2007 | accessdate = April 5, 2012 }}</ref> On September 18 of the following year, the PS3 client became a channel of ] on its launch.<ref>{{cite web | url = http://gizmodo.com/5051558/ps3-news-service-life-with-playstation-now-up-for-download | title = PS3 News Service, Life With Playstation, Now Up For Download | author = Elaine Chow | work = Gizmodo.com | publisher = ] | date = September 18, 2008 | accessdate = April 5, 2012 }}</ref><ref>{{cite web | url = http://folding.typepad.com/news/2008/09/life-with-playstation.html | title = Life with Playstation – a new update to the FAH/PS3 client | author = Vijay Pande | work = Folding@home | publisher = ] | date = September 18, 2008 | accessdate = February 24, 2012 }}</ref> In terms of the types of calculations it can perform, at the time of its introduction the client took the middle ground between a CPU's flexibility and a GPU's speed.<ref name="highper FAQ" /> However, unlike CPUs and GPUs, users cannot perform other activities on their PS3 while running Folding@home.<ref name="cnn ps3" /> The PS3's uniform console environment makes ] easier and makes Folding@home more ].<ref name="Press FAQ" /> The PS3 also has the ability to stream data quickly to its GPU, and is capable of real-time atomic-level visualizations of the current protein dynamics.<ref name="Acc MD on PS3 Cell" /> | ||
| ==== Multi-core processing client ==== | ==== Multi-core processing client ==== | ||
| Folding@home can also utilize the ] capabilities of modern ]s. The ability to use several CPU cores simultaneously allows completion of the overall folding simulation much faster. Working together, these CPU cores complete single Work Units proportionately faster than the standard uniprocessor client,<ref name="SMP FAQ" /> which reduces the traditional difficulties of scaling a large simulation to many separate processors. While this approach is not only scientifically valuable, the resulting publications would not have been possible without this computing power.<ref name="what SMP does" |
Folding@home can also utilize the ] capabilities of modern ]s. The ability to use several CPU cores simultaneously allows completion of the overall folding simulation much faster. Working together, these CPU cores complete single Work Units proportionately faster than the standard uniprocessor client,<ref name="SMP FAQ" /> which reduces the traditional difficulties of scaling a large simulation to many separate processors. While this approach is not only scientifically valuable, the resulting publications would not have been possible without this computing power.<ref name="what SMP does" /> | ||
| In November 2006 first-generation ] (SMP) clients were publicly released for open beta testing, referred to as SMP1.<ref name="highper FAQ" /> These clients used ] (MPI) communication protocols for parallel processing, as at that time the GROMACS cores were ] to be used with multiple threads.<ref name="SMP FAQ" /> This was the first time a distributed computing project had utilized MPI, as it had previously been reserved only for supercomputers,<ref name="new windows client" |
In November 2006 first-generation ] (SMP) clients were publicly released for open beta testing, referred to as SMP1.<ref name="highper FAQ" /> These clients used ] (MPI) communication protocols for parallel processing, as at that time the GROMACS cores were ] to be used with multiple threads.<ref name="SMP FAQ" /> This was the first time a distributed computing project had utilized MPI, as it had previously been reserved only for supercomputers,<ref name="new windows client" /> and SMP1 represented a landmark in the simulation of protein folding.<ref name="what SMP does" /> Although the clients performed well in ]-based operating systems such as ] and Mac's ], they were troublesome under ].<ref name="what SMP does" /><ref name="new windows client" /> On January 24, 2010, SMP2, the second generation of the SMP clients and the successor to SMP1, was released as an open beta and replaced the complex MPI with a more reliable ]-based implementation.<ref name="SMP2 release" /><ref>{{cite web | url = http://folding.typepad.com/news/2009/06/how-does-fah-code-development-and-sysadmin-get-done.html | title = How does FAH code development and sysadmin get done? | author = Vijay Pande | work = Folding@home | publisher = ] | date = June 17, 2009 | accessdate = October 14, 2011 }}</ref> | ||
| SMP2 supported a trial of a special category of "bigadv" Work Units, designed for simulating proteins that are unusually large and computationally intensive and have a great scientific priority. These units originally required a minimum of eight CPU cores,<ref name="bigadv" |
SMP2 supported a trial of a special category of "bigadv" Work Units, designed for simulating proteins that are unusually large and computationally intensive and have a great scientific priority. These units originally required a minimum of eight CPU cores,<ref name="bigadv" /> which was later increased on February 7, 2012 to sixteen CPU cores.<ref>{{cite web | url = http://folding.typepad.com/news/2012/02/update-on-bigadv-16-the-new-bigadv-rollout.html | title = Update on "bigadv-16", the new bigadv rollout | author = Vijay Pande | work = Folding@home | publisher = ] | date = February 7, 2012 | accessdate = February 9, 2012 }}</ref> In addition to these additional hardware requirements over standard SMP2 Work Units, they also require more system resources such as ] and ]. In return, users who run these are rewarded with a 20% increase over SMP2's bonus point system.<ref>{{cite web | url = http://folding.typepad.com/news/2011/07/change-in-the-points-system-for-bigadv-work-units.html | title = Change in the points system for bigadv work units | author = Vijay Pande | work = Folding@home | publisher = ] | date = July 2, 2011 | accessdate = February 24, 2012 }}</ref> The bigadv category allows Folding@home to run particularly demanding simulations on long timescales that had previously required the use of supercomputing ] and could not be performed anywhere else on Folding@home.<ref name="bigadv" /> | ||
| ==== V7 ==== | ==== V7 ==== | ||
| ]. In addition to a variety of controls and user details, V7 also presents Work Unit information, such as its state, calculation progress, ETA, credit points, identification numbers, and description.]] | ]. In addition to a variety of controls and user details, V7 also presents Work Unit information, such as its state, calculation progress, ETA, credit points, identification numbers, and description.]] | ||
| The V7 client is the seventh and latest generation of the Folding@home client software, and is a complete rewrite and unification of the previous clients for ], ] and ] operating systems.<ref name="V7 install guide" |
The V7 client is the seventh and latest generation of the Folding@home client software, and is a complete rewrite and unification of the previous clients for ], ] and ] operating systems.<ref name="V7 install guide" /><ref name="v7 announcement" /> Like its predecessors, V7 can also run Folding@home in the background at a very low priority, allowing other applications to use CPU resources as they need. It is designed to make the installation, start-up, and operation more user-friendly for novices, as well as offers greater scientific flexibility to researchers than previous clients.<ref>{{cite web | url = http://folding.typepad.com/news/2011/03/core-16-for-ati-released-also-note-on-nvidia-gpu-support-for-older-boards.html | title = Core 16 for ATI released; also note on NVIDIA GPU support for older boards | author = Vijay Pande | work = Folding@home | publisher = ] | date = March 31, 2011 | accessdate = September 7, 2011 }}</ref> V7 uses ] for ] so that users can see its development process and provide feedback.<ref name="v7 announcement" /> It was officially released on March 22, 2012.<ref>{{cite web | url = http://folding.typepad.com/news/2012/03/web-page-revamp-and-v7-rollout.html | title = Web page revamp and v7 rollout | author = Vijay Pande | work = Folding@home | publisher = ] | date = March 22, 2012 | accessdate = March 22, 2012 }}</ref> | ||
| V7 consists of four integrated elements. The user typically interacts with V7's open-source ], known as FAHControl. This has Novice, Advanced, and Expert user interface modes, and has the ability to monitor, configure, and control many remote folding clients from a single computer. FAHControl directs FAHClient |
V7 consists of four integrated elements. The user typically interacts with V7's open-source ], known as FAHControl. This has Novice, Advanced, and Expert user interface modes, and has the ability to monitor, configure, and control many remote folding clients from a single computer. FAHControl directs FAHClient – a ] application that in turn manages each FAHSlot (or "slot"). Each slot acts as replacement for the previously distinct Folding@home v6 uniprocessor, SMP, or GPU computer clients, as it can download, process, and upload Work Units independently. The FAHViewer function, modeled after the PS3's viewer, displays a real-time 3D rendering, if available, of the protein currently being processed.<ref name="V7 install guide" /><ref name="v7 announcement" /> | ||
| == Comparison to other molecular systems == | == Comparison to other molecular systems == | ||
| ] is a distributed computing project aimed at ] and is one of the most accurate ] predictors available.<ref>{{cite journal | author = Lensink MF, Méndez R, Wodak SJ | title = Docking and scoring protein complexes: CAPRI 3rd Edition | journal = Proteins | volume = 69 | issue = 4 | pages = 704–18 | year = 2007 | month = December | pmid = 17918726 | doi = 10.1002/prot.21804 }}</ref><ref>{{cite journal | author = Gregory R. Bowman and Vijay S. Pande | title = Simulated tempering yields insight into the low-resolution Rosetta scoring function | journal = Proteins: Structure, Function, and Bioinformatics | year = 2009 | volume = 74 | issue = 3 | pages = 777–88 | doi = 10.1002/prot.22210 | pmid = 18767152 }}</ref> The conformational states from Rosetta's software can be used to initialize a Markov state model as starting points for Folding@home simulations.<ref name="Simulation FAQ" /> Conversely, structure prediction algorithms can be improved from thermodynamic and kinetic models and the sampling aspects of protein folding simulations.<ref>{{cite journal | author = G. R. Bowman and V. S. Pande | title = The Roles of Entropy and Kinetics in Structure Prediction | journal = PLoS ONE | year = 2009 | volume = 4 | issue = 6 | pages = e5840 | doi = 10.1371/journal.pone.0005840 | pmid = 19513117 | pmc = 2688754 | bibcode = 2009PLoSO...4.5840B | editor1-last = Hofmann | editor1-first = Andreas }}</ref> As Rosetta only tries to predict the ], and not how proteins fold, Rosetta@home and Folding@home are complementary and address very different molecular questions.<ref name="Simulation FAQ" /><ref>{{cite web | url = http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1790 | title = Folding@home vs. Rosetta@home | author = Gen_X_Accord, Vijay Pande | work = ] forums | publisher = ] | date = June 11, 2006 | accessdate = April 6, 2012 }}</ref> | ] is a distributed computing project aimed at ] and is one of the most accurate ] predictors available.<ref>{{cite journal | author = Lensink MF, Méndez R, Wodak SJ | title = Docking and scoring protein complexes: CAPRI 3rd Edition | journal = Proteins | volume = 69 | issue = 4 | pages = 704–18 | year = 2007 | month = December | pmid = 17918726 | doi = 10.1002/prot.21804 }}</ref><ref>{{cite journal | author = Gregory R. Bowman and Vijay S. Pande | title = Simulated tempering yields insight into the low-resolution Rosetta scoring function | journal = Proteins: Structure, Function, and Bioinformatics | year = 2009 | volume = 74 | issue = 3 | pages = 777–88 | doi = 10.1002/prot.22210 | pmid = 18767152 }}</ref> The conformational states from Rosetta's software can be used to initialize a Markov state model as starting points for Folding@home simulations.<ref name="Simulation FAQ" /> Conversely, structure prediction algorithms can be improved from thermodynamic and kinetic models and the sampling aspects of protein folding simulations.<ref>{{cite journal | author = G. R. Bowman and V. S. Pande | title = The Roles of Entropy and Kinetics in Structure Prediction | journal = PLoS ONE | year = 2009 | volume = 4 | issue = 6 | pages = e5840 | doi = 10.1371/journal.pone.0005840 | pmid = 19513117 | pmc = 2688754 | bibcode = 2009PLoSO...4.5840B | editor1-last = Hofmann | editor1-first = Andreas }}</ref> As Rosetta only tries to predict the ], and not how proteins fold, Rosetta@home and Folding@home are complementary and address very different molecular questions.<ref name="Simulation FAQ" /><ref>{{cite web | url = http://boinc.bakerlab.org/rosetta/forum_thread.php?id=1790 | title = Folding@home vs. Rosetta@home | author = Gen_X_Accord, Vijay Pande | work = ] forums | publisher = ] | date = June 11, 2006 | accessdate = April 6, 2012 }}</ref> | ||
| ] is a special-purpose supercomputer constructed for molecular dynamics simulations. As of October 2011 Anton and Folding@home are the two most powerful molecular dynamics systems.<ref name="F@h & Anton compared" |
] is a special-purpose supercomputer constructed for molecular dynamics simulations. As of October 2011 Anton and Folding@home are the two most powerful molecular dynamics systems.<ref name="F@h & Anton compared" /> Anton is unique in its ability to produce single ultra-long ] molecular trajectories,<ref name="MSMs & long trajectories" /> such as one in 2010 which reached the millisecond range.<ref>{{cite journal | author = David E. Shaw | title = Millisecond-scale molecular dynamics simulations on Anton | journal = Proceedings of the Conference on High Performance Computing Networking, Storage and Analysis | year = 2009 | issue = 39 | pages = 1–11 | doi = 10.1145/1654059.1654099 | isbn = 978-1-60558-744-8 | author-separator = , | display-authors = 1 | last2 = Bowers | first2 = Kevin J. | last3 = Chow | first3 = Edmond | last4 = Eastwood | first4 = Michael P. | last5 = Ierardi | first5 = Douglas J. | last6 = Klepeis | first6 = John L. | last7 = Kuskin | first7 = Jeffrey S. | last8 = Larson | first8 = Richard H. | last9 = Lindorff-Larsen | first9 = Kresten }}</ref><ref>{{cite journal | author = David E. Shaw | title = Atomic-Level Characterization of the Structural Dynamics of Proteins | journal = Science | year = 2010 | volume = 330 | issue = 6002 | pages = 341–346 | doi = 10.1126/science.1187409 | pmid = 20947758 | bibcode = 2010Sci...330..341S| author-separator = , | display-authors = 1 | last2 = Maragakis | first2 = P. | last3 = Lindorff-Larsen | first3 = K. | last4 = Piana | first4 = S. | last5 = Dror | first5 = R. O. | last6 = Eastwood | first6 = M. P. | last7 = Bank | first7 = J. A. | last8 = Jumper | first8 = J. M. | last9 = Salmon | first9 = J. K. }}</ref> However, Anton does not use Markov state models for analysis. In 2011 the Pande lab constructed a MSM from two 100-] Anton simulations and found alternative folding pathways that were not visible through Anton's traditional analysis. They concluded that there was little difference between MSMs constructed from a limited number of long trajectories or one assembled from many shorter trajectories.<ref name="MSMs & long trajectories" /> In June 2011 Folding@home began additional sampling of an Anton simulation in an effort to better determine how its techniques compare to Anton's methods.<ref>{{cite web | url = http://foldingforum.org/viewtopic.php?f=66&t=18822 | title = Project 7610 & 7611 in Beta | author = TJ Lane (Pande lab member) | work = Folding@home | publisher = ] Group | date = June 6, 2011 | accessdate = February 25, 2012}}{{registration required }}</ref><ref>{{cite web | url = http://fah-web.stanford.edu/cgi-bin/fahproject.overusingIPswillbebanned?p=7610 | title = Project 7610 Description | author = Pande lab | work = Folding@home | accessdate = February 26, 2012 }}</ref> However, unlike Folding@home's shorter trajectories, which are more amendable to distributed computing and other parallelization techniques, longer trajectories do not require adaptive sampling to sufficiently sample the protein's ]. Due to this, it is possible that a combination of Anton's and Folding@home's simulation methods would provide a more thorough sampling of this space.<ref name="MSMs & long trajectories" /> | ||
| == See also == | == See also == | ||
| Line 206: | Line 206: | ||
| == References == | == References == | ||
| {{refs |
{{refs | ||
| | colwidth = 30em | |||
| | refs = | |||
| <ref name="about"> | |||
| {{cite web | url = http://folding.stanford.edu/English/About | title = About Folding@home | author = Pande lab | work = Folding@home | publisher = ] | date = August 2, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="FoldingFAQ"> | |||
| {{cite web | url = http://www.stanford.edu/group/pandegroup/folding/FoldingFAQ.pdf | title = Folding@Home Executive summary | author = Pande lab | work = Folding@home | publisher = ] | accessdate = October 4, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="FAH homepage"> | |||
| {{cite web | url = http://folding.stanford.edu | title = Folding@home homepage | author = Pande lab | work = Folding@home | publisher = ] | year = 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="FAH 4 PS3"> | |||
| {{cite web | url = http://www.scei.co.jp/folding/en/update.html | title = Folding@home for PlayStation3 | work = Folding@home | publisher = Sony | year = 2008 | accessdate = April 5, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Open Source FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-OpenSource | title = Folding@home Open Source FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 2, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Everything about MSMs"> | |||
| {{cite journal | author = V. S. Pande, K. Beauchamp, and G. R. Bowman | title = Everything you wanted to know about Markov State Models but were afraid to ask | journal = Methods | year = 2010 | volume = 52 | issue = 1 | pages = 99–105 | doi = 10.1016/j.ymeth.2010.06.002 | pmc = 2933958 | pmid = 20570730 | |||
| }} | |||
| </ref> | |||
| <ref name="papers"> | |||
| {{cite web | url = http://folding.stanford.edu/English/Papers | title = Recent Results and Research Papers from Folding@home | author = Pande lab | work = Folding@home | publisher = ] | date = July 27, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="NTL9 folding"> | |||
| {{cite journal | author = Vincent A. Voelz, Gregory R. Bowman, Kyle Beauchamp and Vijay S. Pande | title = Molecular simulation of ab initio protein folding for a millisecond folder NTL9(1–39) | journal = Journal of the American Chemical Society | year = 2010 | volume = 132 | issue = 5 | pages = 1526–1528 | doi = 10.1021/ja9090353 | pmid = 20070076 | pmc = 2835335 | |||
| }} | |||
| </ref> | |||
| <ref name="Unraveling mysteries of PF"> | |||
| {{cite journal | author = Heath Ecroyd, John A. Carver | title = Unraveling the mysteries of protein folding and misfolding | format = review | journal = IUBMB Life | year = 2008 | volume = 60 | issue = 12 | pages = 769–774 | doi = 10.1002/iub.117 | pmid =18767168 | |||
| }} | |||
| </ref> | |||
| <ref name="PF then and now"> | |||
| {{cite journal | author = Yiwen Chen, Feng Ding, Huifen Nie, Adrian W. Serohijos, Shantanu Sharma, Kyle C. Wilcox, Shuangye Yin, Nikolay V. Dokholyan | title = Protein folding: Then and now | journal = Archives of Biochemistry and Biophysics | year = 2008 | volume = 469 | issue = 1 | pages = 4–19 | doi = 10.1016/j.abb.2007.05.014 | pmc = 2173875 | pmid = 17585870 | |||
| }} | |||
| </ref> | |||
| <ref name="from the TT to organism"> | |||
| {{cite journal | author = Leila M Luheshi, Damian Crowther, Christopher Dobson | title = Protein misfolding and disease: from the test tube to the organism | journal = Current Opinion in Chemical Biology | year = 2008 | volume = 12 | issue = 1 | pages = 25–31 | doi = 10.1016/j.cbpa.2008.02.011 | pmid = 18295611 | |||
| }} | |||
| </ref> | |||
| <ref name="how well can simulation predict"> | |||
| {{cite journal | author = C. D. Snow, E. J. Sorin, Y. M. Rhee, and V. S. Pande. | title = How well can simulation predict protein folding kinetics and thermodynamics? | format = review | journal = Annual Reviews of Biophysics | year = 2005 | volume = 34 | pages = 43–69 | doi = 10.1146/annurev.biophys.34.040204.144447 | pmid = 15869383 | |||
| }} | |||
| </ref> | |||
| <ref name="taming folding complexity"> | |||
| {{cite journal | author = G. Bowman, V. Volez, and V. S. Pande | title = Taming the complexity of protein folding | journal = Current Opinion in Structural Biology | year = 2011 | volume = 21 | issue = 1 | pages = 4–11 | doi = 10.1016/j.sbi.2010.10.006 | pmc = 3042729 | pmid = 21081274 | |||
| }} | |||
| </ref> | |||
| <ref name="Simulation FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-Simulation | title = Folding@home Simulation FAQ | author = TJ Lane, Gregory Bowman, Robert McGibbon, Christian Schwantes, Vijay Pande, and Bruce Borden | work = Folding@home | publisher = ] | date = September 10, 2012 | accessdate = September 10, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="adaptive sampling of MSMs"> | |||
| {{cite journal | author = Gregory R. Bowman, Daniel L. Ensign, and Vijay S. Pande | title = Enhanced Modeling via Network Theory: Adaptive Sampling of Markov State Models | journal = Journal of Chemical Theory and Computation | year = 2010 | volume = 6 | issue = 3 | pages = 787–794 | doi = 10.1021/ct900620b | |||
| }} | |||
| </ref> | |||
| <ref name="Protein Misfolding Diseases"> | |||
| {{cite journal | author = Vittorio Bellotti and Monica Stoppini | title = Protein Misfolding Diseases | journal = The Open Biology Journal | year = 2009 | volume = 2 | pages = 228–234 | url = http://www.benthamscience.com/open/tobioj/articles/V002/SI0161TOBIOJ/228TOBIOJ.pdf | |||
| }} | |||
| </ref> | |||
| <ref name="diseases FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-Diseases | title = Folding@home Diseases Studied FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = May 30, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Collier Virology"> | |||
| {{cite book | last1 = Collier | first1 = Leslie | last2 = Balows | first2 = Albert | last3 = Sussman | first3 = Max | year = 1998 | chapter = | title = Topley and Wilson's Microbiology and Microbial Infections | edition = ninth | volume = 1{{nobold|, ''Virology''}} | editor1-last = Mahy | editor1-first = Brian | editor2-last = Collier | editor2-first = Leslie | publisher = Arnold | location = London | isbn = 978-0-340-66316-5 | pages = 75–91 | |||
| }} | |||
| </ref> | |||
| <ref name="Comp-aided drug design"> | |||
| {{cite journal | author = Chun Song, Shen Lim, Joo Tong | title = Recent advances in computer-aided drug design | format = review | journal = Briefings in Bioinformatics | year = 2009 | volume = 10 | issue = 5 | pages = 579–91 | doi = 10.1093/bib/bbp023 | pmid = 19433475 | |||
| }} | |||
| </ref> | |||
| <ref name="Press FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-Press | title = Folding@Home Press FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | year = 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Main FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-main | title = Folding@home Main FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 18, 2011 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="F@H & Simbios"> | |||
| {{cite web | url = http://folding.typepad.com/news/2008/04/foldinghome-and.html | title = Folding@home and Simbios | author = Vijay Pande | work = Folding@home | publisher = ] | date = April 23, 2008 | accessdate = November 9, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="papers for free"> | |||
| {{cite web | url = http://foldingforum.org/viewtopic.php?f=16&t=19643&p=197898#p197898 | title = Re: Suggested Changes to F@h Website | author = Vijay Pande | work = Folding@home | publisher = ] Group | date = October 25, 2011 | accessdate = October 25, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="biologists think bigger"> | |||
| {{cite journal | author = Caroline Hadley | title = Biologists think bigger | journal = EMBO Reports | year = 2004 | volume = 12 | issue = 5 | pages = 236–238 | doi = 10.1038/sj.embor.7400108 | url = http://www.nature.com/embor/journal/v5/n3/full/7400108.html | |||
| }} | |||
| </ref> | |||
| <ref name="PM&N review"> | |||
| {{cite journal | author = Claudio Soto, Lisbell D. Estrada | title = Protein Misfolding and Neurodegeneration | format = review | journal = Archives of Neurology | year = 2008 | volume = 65 | issue = 2 | pages = 184–189 | doi = 10.1001/archneurol.2007.56 | pmid = 18268186 | |||
| }} | |||
| </ref> | |||
| <ref name="AB assembly and AD"> | |||
| {{cite journal | author = Robin Roychaudhuri, Mingfeng Yang, Minako M. Hoshi and David B. Teplow | title = Amyloid β-Protein Assembly and Alzheimer Disease | journal = Journal of Biological Chemistry | format = minireview | year = 2008 | volume = 284 | issue = 8 | pages = 4749–53 | doi = 10.1074/jbc.R800036200 | pmid = 18845536 | |||
| }} | |||
| </ref> | |||
| <ref name="Simulating oligomerization"> | |||
| {{cite journal | author = Nicholas W. Kelley, V. Vishal, Grant A. Krafft, and Vijay S. Pande. | title = Simulating oligomerization at experimental concentrations and long timescales: A Markov state model approach | journal = Journal of Chemical Physics | year = 2008 | volume = 129 | issue = 21 | page = 214707 | doi = 10.1063/1.3010881 | pmid = 19063575 | pmc = 2674793 | bibcode = 2008JChPh.129u4707K | |||
| }} | |||
| </ref> | |||
| <ref name="Novick2011"> | |||
| {{cite journal | author = P. Novick, J. Rajadas, C.W. Liu, N. W. Kelley, M. Inayathullah, and V. S. Pande | title = Rationally Designed Turn Promoting Mutation in the Amyloid-β Peptide Sequence Stabilizes Oligomers in Solution | journal = PLoS ONE | year = 2011 | volume = 6 | issue = 7 | pages = e21776 | doi = 10.1371/journal.pone.0021776 | pmc = 3142112 | pmid = 21799748 | editor1-last = Buehler | editor1-first = Markus J. | |||
| }} | |||
| </ref> | |||
| <ref name="MSM applications"> | |||
| {{cite journal | author = Gregory R Bowman, Xuhui Huang, and Vijay S Pande | title = Network models for molecular kinetics and their initial applications to human health | format = review | journal = Cell Research | year = 2010 | volume = 20 | issue = 6 | pages = 622–630 | doi = 10.1038/cr.2010.57 | pmid = 20421891 | |||
| }} | |||
| </ref> | |||
| <ref name="Huntingtin headpiece structure"> | |||
| {{cite journal | author = Nicholas W. Kelley, Xuhui Huang, Stephen Tam, Christoph Spiess, Judith Frydman and Vijay S. Pande | title = The predicted structure of the headpiece of the Huntingtin protein and its implications on Huntingtin aggregation | journal = Journal of Molecular Biology | year = 2009 | volume = 388 | issue = 5 | pages = 919–27 | doi = 10.1016/j.jmb.2009.01.032 | pmid = 19361448 | pmc = 2677131 | |||
| }} | |||
| </ref> | |||
| <ref name="New drug design methods"> | |||
| {{cite web | url = http://folding.typepad.com/news/2012/02/new-methods-for-computational-drug-design.html | title = New methods for computational drug design | author = Vijay Pande | work = Folding@home | publisher = ] | date = February 27, 2012 | accessdate = April 1, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="POP comp"> | |||
| {{cite journal | author = Guha Jayachandran, M. R. Shirts, S. Park, and V. S. Pande | title = Parallelized-Over-Parts Computation of Absolute Binding Free Energy with Docking and Molecular Dynamics | journal = Journal of Chemical Physics | year = 2006 | volume = 125 | issue = 8 | page = 084901 | doi = 10.1063/1.2221680 | pmid = 16965051 | bibcode = 2006JChPh.125h4901J | |||
| }} | |||
| </ref> | |||
| <ref name="drug target search"> | |||
| {{cite web | url = http://folding.typepad.com/news/2012/07/searching-for-new-drug-targets.html | title = Searching for new drug targets | author = Gregory Bowman | work = Folding@home | publisher = ] | date = July 23, 2012 | accessdate = September 27, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="FLOPS FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-flops | title = Folding@home FLOP FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = April 4, 2009 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="osstats"> | |||
| {{cite web | url = http://fah-web.stanford.edu/cgi-bin/main.py?qtype=osstats | title = Client Statistics by OS | author = Pande lab | work = Folding@home | publisher = Stanford University | date = updated daily | accessdate = September 13, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="PS3 FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-PS3 | title = PS3 FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = May 30, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Biotech 27"> | |||
| {{cite web | url = http://castroller.com/Podcasts/FuturesInBiotech/249153 | title = Futures in Biotech 27: Folding@home at 1.3 Petaflops | format = Interview, webcast | work = Castroller.com | publisher = CastRoller | date = December 28, 2007 | accessdate = April 5, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="past 4 petaFLOPS"> | |||
| {{cite web | url = http://team52735.blogspot.com/2008_09_29_archive.html | title = Increase in 'active' PS3 folders pushes Folding@home past 4 Petaflops! | work = team52735.blogspot.com | publisher = ] | date = September 29, 2008 | accessdate = August 23, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="FAH Stats doc"> | |||
| {{cite web | url = https://docs.google.com/spreadsheet/ccc?key=0AlVJhzLM5aDCdFJTTFVPSXVWTFh3QVdTSGxvUWJpY1E#gid=0 | title = Folding@home Stats | author = screen317, others | publisher = ] | accessdate = August 23, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="6 petaFLOPS"> | |||
| {{cite web | url = http://foldingforum.org/viewtopic.php?f=16&t=20011#p198840 | title = Six Native PetaFLOPS | author = Jesse Victors | work = Folding@home | publisher = ] Group | date = November 10, 2011 | accessdate = November 11, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="Points FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-Points | title = Folding@home Points FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 20, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="PointsNew FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-PointsNew | title = Folding@home Points FAQ (New Benchmark Machine – January 2010) | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = August 16, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="SMP2 release"> | |||
| {{cite web | url = http://foldingforum.org/viewtopic.php?f=24&t=13038#p127406 | title = upcoming release of SMP2 cores | author = Peter Kasson (Pande lab member) | work = Folding@home | publisher = ] Group | date = January 24, 2010 | accessdate = September 30, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="lessons from 8 years"> | |||
| {{cite journal | author = Adam Beberg, Daniel Ensign, Guha Jayachandran, Siraj Khaliq, Vijay Pande | title = Folding@home: Lessons From Eight Years of Volunteer Distributed Computing | journal = Parallel & Distributed Processing, IEEE International Symposium | year = 2009 | pages = 1–8 | doi = 10.1109/IPDPS.2009.5160922 | issn = 1530-2075 | url = http://www.hicomb.org/papers/HICOMB2009-13.pdf | isbn = 978-1-4244-3751-1 | |||
| }} | |||
| </ref> | |||
| <ref name="SMP FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-SMP | title = Folding@home SMP FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = June 11, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="Acc. MD on GPU"> | |||
| {{cite journal | author = M. S. Friedrichs, P. Eastman, V. Vaidyanathan, M. Houston, S. LeGrand, A. L. Beberg, D. L. Ensign, C. M. Bruns, V. S. Pande | title = Accelerating Molecular Dynamic Simulation on Graphics Processing Units | journal = Journal of Computational Chemistry | year = 2009 | volume = 30 | issue = 6 | pages = 864–72 | doi = 10.1002/jcc.21209 | pmid = 19191337 | pmc = 2724265 | |||
| }} | |||
| </ref> | |||
| <ref name="Uni Guide"> | |||
| {{cite web | url = http://folding.stanford.edu/English/WinUNIGuide | title = Windows Uniprocessor Client Installation Guide | author = Pande lab | work = Folding@home | publisher = ] | date = February 10, 2011 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="license"> | |||
| {{cite web | url = http://folding.stanford.edu/English/License | title = Folding@home Distributed Computing Client | author = Pande lab | work = Folding@home | publisher = ] | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="uninstall"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-Uninstall | title = Uninstalling Folding@home FAQ | author = Pande lab | work = Folding@home | publisher = ] | date = May 30, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="highper FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-highperformance | title = High Performance FAQ | author = Pande lab | work = Folding@home | publisher = ] | format = FAQ | date = May 30, 2012 | accessdate = August 20, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="ATI FAQ"> | |||
| {{cite web | url = http://folding.stanford.edu/English/FAQ-ATI | title = ATI FAQ | format = FAQ | author = Pande lab | work = Folding@home | publisher = ] | date = March 18, 2011 | accessdate = March 21, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="GPU3 prep"> | |||
| {{cite web | url = http://folding.typepad.com/news/2010/04/prepping-for-the-gpu3-rolling-new-client-and-nvidia-fah-gpu-clients-will-need-cuda-22-or-later.html | title = Prepping for the GPU3 rolling: new client and NVIDIA FAH GPU clients will (in the future) need CUDA 2.2 or later | author = Vijay Pande | work = Folding@home | publisher = ] | date = April 24, 2010 | accessdate = September 8, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="Acc MD on PS3 Cell"> | |||
| {{cite journal | author = Edgar Luttmann, Daniel L. Ensign, Vishal Vaidyanathan, Mike Houston, Noam Rimon, Jeppe Øland, Guha Jayachandran, Mark Friedrichs, Vijay S. Pande | title = Accelerating Molecular Dynamic Simulation on the Cell processor and PlayStation 3 | journal = Journal of Computational Chemistry | year = 2008 | volume = 30 | issue = 2 | pages = 268–274 | doi = 10.1002/jcc.21054 | pmid = 18615421 | |||
| }} | |||
| </ref> | |||
| <ref name="cnn ps3"> | |||
| {{cite web | url = http://edition.cnn.com/2006/TECH/fun.games/09/18/playstation.folding/ | title = PlayStation's serious side: Fighting disease | author = David E. Williams | publisher = CNN | date = October 20, 2006 | accessdate = April 5, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="what SMP does"> | |||
| {{cite web | url = http://folding.typepad.com/news/2008/06/what-does-the-smp-core-do.html | title = What does the SMP core do? | author = Vijay Pande | work = Folding@home | publisher = ] | date = June 15, 2008 | accessdate = September 7, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="new windows client"> | |||
| {{cite web | url = http://folding.typepad.com/news/2008/03/new-windows-cli.html | title = New Windows client/core development (SMP and classic clients) | author = Vijay Pande | work = Folding@home | publisher = ] | date = March 8, 2008 | accessdate = September 30, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="bigadv"> | |||
| {{cite web | url = http://foldingforum.org/viewtopic.php?t=10697 | title = new release: extra-large work units | author = Peter Kasson (Pande lab member) | work = Folding@home | publisher = ] Group | date = July 15, 2009 | accessdate = October 9, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="V7 install guide"> | |||
| {{cite web | url = http://folding.stanford.edu/English/WinGuide | title = Windows (FAH V7) Install Guide | author = Pande lab | work = Folding@home | publisher = ] | date = March 23, 2012 | accessdate = March 21, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="v7 announcement"> | |||
| {{cite web | url = http://folding.typepad.com/news/2011/03/client-version-7-now-in-open-beta.html | title = Client version 7 now in open beta | author = Vijay Pande | work = Folding@home | publisher = ] | date = March 29, 2011 | accessdate = August 14, 2011 | |||
| }} | |||
| </ref> | |||
| <ref name="F@h & Anton compared"> | |||
| {{cite web | url = http://folding.typepad.com/news/2011/10/comparison-between-fah-and-antons-approaches.html | title = Comparison between FAH and Anton's approaches | author = Vijay Pande | work = Folding@home | publisher = ] | date = October 13, 2011 | accessdate = February 25, 2012 | |||
| }} | |||
| </ref> | |||
| <ref name="MSMs & long trajectories"> | |||
| {{cite journal | author = Thomas J. Lane, Gregory R. Bowman, Kyle A Beauchamp, Vincent Alvin Voelz, and Vijay S. Pande | title = Markov State Model Reveals Folding and Functional Dynamics in Ultra-Long MD Trajectories | journal = Journal of the American Chemical Society | year = 2011 | volume = 133 | issue = 45 | pages = 18413–9 | doi = 10.1021/ja207470h | pmid = 21988563 | pmc = 3227799 | |||
| }} | |||
| </ref> | |||
| }} | |||
| == External links == | == External links == | ||
| * | * | ||
| * | * | ||
Revision as of 07:24, 17 September 2012
 | |
| Original author(s) | Vijay Pande |
|---|---|
| Developer(s) | Pande laboratory, Sony, Nvidia, ATI, Cauldron Development |
| Initial release | October 1, 2000 |
| Stable release | Windows, Linux: 7.1.52 Mac OS X: 6.29.3 PlayStation 3: 1.4 |
| Operating system | Microsoft Windows, Mac OS X, Linux |
| Platform | Cross-platform |
| Available in | English |
| Type | Distributed computing |
| License | Partially GPL, partially proprietary |
| Website | folding |
Folding@home (FAH or F@h) is a distributed computing project for simulation of protein folding, computational drug design, and other molecular dynamics for disease research. Folding@home is powered by the idle processing resources of thousands of personal computers and PlayStation 3s from volunteers who have installed the software on these systems. The project primarily attempts to determine the mechanisms of protein folding, (the process by which proteins reach their final three-dimensional structure) and the reasons behind protein misfolding. This is of significant academic interest and has major implications for medical research into Alzheimer's disease, Huntington's disease, and many forms of cancer, among other diseases. To a lesser extent, Folding@home also tries to predict a protein's final structure and determine how other molecules may interact with it, which has applications in drug design. Folding@home is developed and operated by the Pande laboratory at Stanford University, under the leadership of Vijay Pande, and is shared by various scientific institutions and research laboratories across the world in a collaboration known as the Folding@home Consortium.
The project uses statistical simulation methodology that represents a paradigm shift from traditional computational approaches. As part of the project's client-server architecture, the volunteered machines receive simulation Work Units, complete them, and return them to database servers where they are compiled into an overall simulation. Volunteers can track their contributions on the Folding@home website, which can make participation competitive and encourages long-term involvement. The project has pioneered the uses of GPUs, PlayStation 3s, and Message Passing Interface (used for computing on multi-core processors) for distributed computing and scientific research.
Folding@home remains one of the world's fastest computing systems, and currently operates at a computational performance nearly equal to all distributed computing projects under BOINC combined. The project is also the world's most powerful molecular dynamics simulator. This performance from its large-scale computing network has allowed researchers to run computationally expensive atomic-level simulations thousands of times longer than previously achieved. Since its launch on October 1, 2000, the Pande lab has produced 100 scientific research papers as a direct result of the project. These simulations have demonstrated accuracy compared to experimental observations.
Project significance
Further information: Protein folding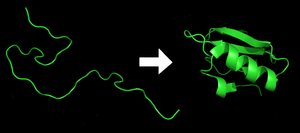
Proteins are an essential component to many biological functions and participate in virtually all processes within biological cells. They often act as enzymes, performing biochemical reactions including cell signaling, molecular transportation, and cellular regulation. As structural elements, some proteins act as a type of skeleton for cells, and as antibodies, other proteins participate in the immune system. Before a protein can take on these roles, it must fold into a functional three-dimensional structure, a process that often occurs spontaneously and is dependent on interactions within its amino acid sequence. Protein folding is driven by the search to find the most energetically favorable conformation of the protein, i.e. its native state. Thus, understanding protein folding is critical to understanding what a protein does and how it works, and is considered a "holy grail" of computational biology. Despite folding occurring within a crowded cellular environment, it typically proceeds smoothly. However, due to a protein's chemical properties or other factors, proteins may misfold — that is, fold down the wrong pathway and end up misshapen. Unless cellular mechanisms are capable of destroying or refolding such misfolded proteins, they can subsequently aggregate and cause a variety of debilitating diseases. Laboratory experiments studying these processes can be limited in scope and atomic detail, leading scientists to use physics-based computational models that, when complementing experiments, seek to provide a more complete picture of protein folding, misfolding, and aggregation.
Due to the complexity of proteins' conformation space and limitations in computational power, all-atom molecular dynamics simulations have been severely limited in the timescales which they can study. While most proteins typically fold in the order of milliseconds, prior to 2010 simulations could only reach nanosecond to microsecond timescales. General-purpose supercomputers have been used to simulate protein folding, but such systems are intrinsically expensive and typically shared between many different research groups, and because the computations in kinetic models are serial in nature, strong scaling of traditional molecular simulations to these architectures is exceptionally difficult. Additionally, as the protein folding process is stochastic, a limited number of long simulations are not sufficient for comprehensive views of protein folding.
Protein folding does not occur in a single step. Instead, proteins spend the majority of their folding time – nearly 96% in some cases – "waiting" in various intermediate conformational states, each a local thermodynamic free energy minimum in the protein's energy landscape. Through a process known as adaptive sampling, these conformations are used by Folding@home as starting points for a set of simulations trajectories. As the simulations discover more conformations, the trajectories are restarted from them, and a Markov state model (MSM) is gradually created from this cyclic process. MSMs are discrete-time master equation models which map out a biomolecule's conformational and energy landscape by describing its set of distinct structures and the transition rates between them. The adaptive sampling Markov state model approach significantly increases the efficiency of simulation as it avoids computation inside the local energy minimum itself, and is amenable to distributed computing (including on GPUGRID) as it allows for the statistical aggregation of short, independent simulation trajectories. The amount of time it takes to construct a Markov state model is inversely proportional to the number of parallel simulations run, i.e. the number of processors available. In other words, it achieves near-linear parallelization, leading to an approximately four orders of magnitude reduction in overall serial calculation time. A completed MSM illustrates the probability of folding events and pathways from the protein's phase space, may contain tens of thousands of states, and through kinetic clustering of the conformations it can represent these states at an arbitrary resolution. Researchers can use these MSMs to reveal how proteins misfold and to quantitatively compare simulations with experiments. Between 2000 and 2010, the timescales over which Folding@home simulates protein folding have increased by six orders of magnitude.
In 2002, Folding@home used Markov state models to complete approximately a million CPU days of simulations over the span of several months, and in 2011, MSMs parallelized another simulation that required an aggregate 10 million CPU hours of computation. In January 2010, Folding@home used MSMs to simulate the dynamics of the slow-folding 32-residue NTL9 protein out to 1.52 milliseconds, a timescale consistent with experimental folding rate predictions but a thousand times longer than previously achieved. The model consisted of many individual trajectories, each two orders of magnitude shorter. This was the first demonstration that MSMs were capable of statistically capturing folding events that could not be seen by conventional simulation methods. In 2010, Folding@home researcher Greg Bowman was awarded the Thomas Kuhn Paradigm Shift Award from the American Chemical Society for the instrumental development of the open-source MSMBuilder software and for attaining quantitative agreement between theory and experiment. For his work, Pande was awarded the 2012 Michael and Kate Bárány Award for Young Investigators for "developing field-defining and field-changing computational methods to produce leading theoretical models for protein and RNA folding" as well as the 2006 Irving Sigal Young Investigator Award "for his unique approach to employing advances in algorithms that make optimal use of distributed computing, which places his efforts at the cutting edge of simulations. The results have stimulated a re-examination of the meaning of both ensemble and single-molecule measurements, making Dr. Pande’s efforts pioneering contributions to simulation methodology."
Biomedical research
Protein folding is naturally tightly regulated to ensure that it proceeds smoothly. The failure of a protein to fold correctly can result in the development of a variety of diseases including alpha 1-antitrypsin deficiency, Alzheimer's disease, autism, cancer, Creutzfeldt–Jakob disease, cystic fibrosis, Huntington's disease, Mad Cow, Parkinson's disease, sickle-cell anaemia, and type II diabetes. Once it is understood how a protein misfolds, therapeutic intervention can follow, which can use engineered molecules to alter the production of a certain protein, to help destroy a misfolded protein, or to assist in the folding process. Cellular infection by viruses such as HIV and influenza also involve folding events within cellular membranes. Computer-assisted drug design has the potential to expedite and lower the costs of drug discovery. The combination of computational molecular modeling and experimental analysis has the possibility of fundamentally shaping the future of molecular medicine and the rational design of therapeutics. Folding@home is dedicated to producing significant amounts of results about protein folding, the diseases that result from protein misfolding, and the development of novel computational methods for drug design. The goal of the first five years of the project was to make significant advances in understanding folding, while the current goal is to understand misfolding and related disease, especially Alzheimer's disease.
The simulations run on Folding@home used in conjunction with laboratory experiments, but researchers can use it to study how folding in vitro differs from folding in native cellular environments. This is advantageous in studying aspects of folding, misfolding, and its relationship to disease that are exceptionally difficult to observe experimentally. For example, in 2011 Folding@home continued simulations of folding inside a ribosomal exit tunnel, to help scientists better understand how natural confinement and crowding might influence the folding process. Furthermore, scientists typically employ chemical denaturants to unfold proteins from their stable native state. It is not generally known how the denaturant affects the protein's refolding, and it is difficult to experimentally determine if these denatured states contain residual structures which may influence folding behavior. In 2010, Folding@home simulated the unfolded states of Protein L, and predicted the collapse rate in strong agreement with experimental results.
The Pande lab is a non-profit organization and does not sell the results generated by Folding@home. The large data sets from the project are freely available for other researchers to use upon request and some can be accessed from the Folding@home website. The Pande lab has collaborated with other molecular dynamics systems such as the Blue Gene supercomputer, and they share Folding@home's key software with other researchers, so that the algorithms which benefited Folding@home may aid other scientific areas. In 2011 they released the open-source Copernicus software, which is based on Folding@home's MSM and other parallelization techniques and aims to significantly improve the efficiency and scaling of molecular simulations on large computer clusters or supercomputers. Summaries of all of the scientific findings from Folding@home are posted on the Folding@home website after publication. The full publications are available online from an academic library.
Alzheimer's disease

Alzheimer's is an incurable neurodegenerative disease which most most often affects the elderly. It accounts for more than half of all cases of dementia, and as of 2008 it affects more than 24 million people worldwide, with 4.6 million new cases reported each year. Its exact cause remains unknown, but the disease is identified as a protein misfolding disease and is associated with toxic aggregations of the amyloid beta (Aβ) peptide, a fragment of the larger amyloid precursor protein. High concentrations of misfolded Aβ42 causes protein oligomer growth leading to aggregation that in turn contributes to Aβ misfolding. This cyclic process appears to be toxic and leads to neuronal cell death. The oligomer aggregates then collect into dense nontoxic formations known as senile plaques, a pathological marker of Alzheimer's disease. Due to the heterogeneous nature of Aβ oligomer aggregates, experimental techniques such as x-ray crystallography and NMR have had difficulty characterizing their structures. Moreover, atomistic simulations are extremely computationally demanding due to their size and complexity.
Disabling Aβ aggregation using small molecules is regarded as a promising approach to the development of therapeutic drugs for treating Alzheimer's patients. The Pande lab is focusing their research on Alzheimer's with the goal of predicting the aggregate structure and how it develops for drug design approaches as well as developing methods to stop the aggregation process. In 2008 Folding@home simulated the dynamics of Aβ in atomic detail over timescales of the order of tens of seconds. This was significant as previous simulations were about six orders of magnitude shorter. Researchers used the resulting Markov state model to identify a β-Hairpin that was a major source of molecular interactions within the structure. This study helped prepare the Pande lab for future aggregation studies and for further research to find a small peptide which may stabilize the aggregation process. In the same year, Folding@home found several small drug candidates which appear to inhibit the toxicity of Aβ. In 2010, in close cooperation with the Nanomedicine Center for Protein Folding, these drug leads went from the test tube to testing on biological tissue. In 2011, Folding@home completed simulations of several mutations of Aβ that appear to stabilize the aggregate formation, which could aid in the development of therapeutic drug approaches to the disease as well as greatly assisting with experimental NMR spectroscopy studies of the oligomers. Later that year, Folding@home began simulations of various Aβ fragments in order to determine how various natural enzymes affect the structure and folding of Aβ.
Huntington's disease
Huntington's disease is a neurodegenerative genetic disorder that is also associated with protein misfolding and aggregation. Excessive repeats of the glutamine amino acid at the N-terminus of the Huntingtin protein cause aggregation, and although the behavior of the repeats is not completely understood, it does lead to the cognitive decline associated with the disease. As with other aggregates, there is difficulty in experimentally determining its structure. Scientists are using Folding@home to study Huntingtin protein aggregate structure as well as to predict how the aggregate forms, assisting with rational drug design approaches to stop the aggregate formation. The N17 fragment of the Huntingtin protein accelerates this aggregation, and while there have been several proposed mechanisms, its exact role in this process remains largely unknown. Folding@home has simulated this and other fragments in order to elucidate their roles in the disease. Since 2008, its drug design approaches for Alzheimer's disease have been applied to Huntington's, and in 2010, Folding@home researcher Veena Thomas proposed a novel therapeutic strategy for Huntington's which may be funded by the National Institutes of Health. This strategy could be used to bring the results from Folding@home directly to a therapeutic drug.
Cancer
More than half of all known cancers involve mutations of p53, a tumor suppressor protein present in every cell which regulates the cell cycle and signals for cell death in the event of damage to DNA. Specific mutations in p53 can disrupt these functions, allowing an abnormal cell to continue growing unchecked, resulting in the development of tumors. These deleterious mutations may differ between the various types and locations of cancer, and analysis of these mutations is important for understanding the root causes of p53-related cancers. In 2004, Folding@home was used to perform the first molecular dynamics study of the refolding of p53's protein dimer in explicit water which revealed insights that were previously unobtainable, and from it produced the first peer reviewed publication on cancer from a distributed computing project. The following year, it powered a new method to identify the amino acids crucial for the stability of a given protein, which was then used to study mutations of p53. The method demonstrated reasonable success in identifying cancer-promoting mutations and determined the effects of specific mutations which could not otherwise be measured experimentally. Following these studies, the Pande lab expanded their efforts to other p53-related diseases.
Folding@home is also being used to study protein chaperones, heat shock proteins which play essential roles in cell survival by assisting with the folding of other proteins inside the crowded and chemically stressful intracellular environment. Rapidly growing cancer cells rely on specific chaperones, and some chaperones play key roles in chemotherapy resistance. Inhibiting these specific chaperones are seen as potential modes of action for efficient antineoplastic drugs or for reducing the spread of cancer. Using Folding@home and working closely with the Protein Folding Center, the Pande lab hopes to find a drug which inhibits those chaperones involved in cancerous cells. Researchers are also using Folding@home to study other molecules related to cancer, such as the enzyme Src kinase and certain forms of the Engrailed homeodomain. In 2011, Folding@home began simulations of the dynamics of the small knottin protein EETI, which can identify carcinomas in imaging scans by binding to surface receptors of cancer cells. From simulations of this protein, they hope to accelerate research efforts to modify it to identify other diseases or to bind to drugs.
Interleukin 2 (IL-2) is a protein which plays crucial roles in helping T cells of the immune system attack pathogens and tumors. Unfortunately, its use as a cancer treatment is restricted due to serious side effects such as pulmonary edema. IL-2 binds to these pulmonary cells differently than it does to T cells, so IL-2 research involves understanding the differences between these binding mechanisms. In 2012, Folding@home assisted with the discovery of a form of IL-2 which is three hundred times more effective in its immune system role but carries fewer side effects. In experiments, this altered form significantly outperformed natural IL-2 in impeding tumor growth. Pharmaceutical companies have expressed interest in the mutant molecule, and the National Institutes of Health is testing it against a large variety of tumor models in the hopes of accelerating its development as a therapeutic.
Osteogenesis imperfecta
Osteogenesis imperfecta, also known as brittle bone disease, is a incurable genetic bone disorder which can be lethal. Those with the disease are unable to make functional connective bone tissue. This is most commonly due to a mutation in Type-I collagen, which fulfills a variety of structural roles and is the most abundant protein in mammals. The mutation causes a deformation in collagen's triple helix structure, which if not naturally destroyed, leads to abnormal and weakened bone tissue. In 2005, Folding@home tested a new quantum mechanical technique that improved upon previous simulations methods, and which may be useful for future computational studies of collagen. Although researchers have used Folding@home to study collagen folding and misfolding, the interest stands as a pilot project compared to Alzheimer's and Huntington's research.
Viruses
Folding@home is assisting in research towards preventing certain viruses such as influenza and HIV from recognizing and entering biological cells. Influenza in particular has been responsible for periodic high-mortality pandemics, such as the 1918 flu pandemic which may have killed upwards of 100 million people worldwide. Membrane fusion is an essential event for viral infection and involves conformational changes of viral fusion proteins and protein docking. A virus may enter after this process, or a virus may envelop itself in the cell's membrane. Membrane fusion is also crucial to a wide range of biological functions and controlling it has pharmaceutical implications, but the exact molecular mechanisms behind fusion remain largely unknown. Fusion events may involve the interactions of over a half million atoms for hundreds of microseconds. This complexity and timescale makes standard computer simulations exceptionally computationally demanding, so they are typically limited to about ten thousand atoms over tens of nanoseconds: a difference of several orders of magnitude. Moreover, such complex mechanisms are difficult to analyse experimentally. However, in 2006 scientists applied Markov state models and the Folding@home network to gain detailed mechanistic insights into the fusion process.
Using Folding@home for detailed simulations of vesicle fusion, in 2007 the Pande lab introduced a new technique for measuring fusion intermediate topology. In 2009, researchers used Folding@home to study mutations of influenza hemagglutinin, a protein that attaches a virus to its target cell and assists with viral entry. Mutations to hemagglutinin affect the binding affinity to the cell surface receptor glycan of a target species, which determines the infectivity of the virus strain to that species. Knowledge of the effects of hemagglutinin mutations assists in the development of antiviral drugs. In 2011 Folding@home began simulations of the dynamics of the enzyme RNase H, a key component of HIV, in the hopes of designing drugs to deactivate it. As of 2012, Folding@home continues to simulate the folding and interactions of hemagglutinin, complementing experimental studies at the University of Virginia.
Drug design
Drugs function by binding to specific locations on target molecules and causing a certain desired change. Ideally, a drug should act very specifically and bind only to its target without interfering with other biological functions. However, it is difficult to precisely determine where and how tightly two molecules will bind. Due to limitations in computational power, current in silico approaches usually have to trade speed for accuracy; e.g. use rapid protein docking methods instead of computationally expensive free energy calculations. Folding@home's computational performance allows researchers to use both techniques, and evaluate their efficiency and reliability. Computer-assisted drug design has the potential to expedite and lower the costs of drug discovery. In 2010, Folding@home used MSMs and free energy calculations to predict the native structure of the villin protein to within 1.8 Å RMSD from the crystalline structure experimentally determined through X-ray crystallography. This may be important to future protein structure prediction approaches, including for intrinsically unstructured proteins. Scientists have used Folding@home to research drug resistance by studying vancomycin, an antibiotic of "last resort", and beta-lactamase, a protein that can break down antibiotics like penicillin. They hope to be better able to design drugs to deactivate them.
Chemical activity occurs along a protein's active site. Traditional drug design approaches involve tightly binding to this site and blocking its activity, under the assumption that the target protein exists in a single rigid structure. However, this approach only works for approximately 15% of all proteins. Proteins contain allosteric sites which, when bound to by small molecules, can alter a protein's conformation and ultimately affect the protein's activity. These sites are attractive drug targets, but locating them is very computationally expensive. In 2012, Folding@home and MSMs were used to identify allosteric site in three medically relevant proteins: ß lactamase, interleukin-2, and RNase H.
Approximately half of all known antibiotics interfere with the workings of a bacteria's ribosome, a large and complex biochemical machine that performs protein biosynthesis by translating messenger RNA into proteins. Macrolide antibiotics clog the ribosome's exit tunnel, preventing synthesis of essential bacterial proteins. In 2007 the Pande lab received a grant to study and design new antibiotics. In 2008 they used Folding@home to study the interior of this tunnel and how specific molecules may affect it. The full structure of the ribosome has only been recently determined, and Folding@home has also simulated ribosomal proteins, as many of their functions remain largely unknown. Ribosomal research has helped the Pande lab prepare for larger and more complex biomedical problems.
Participation

In addition to reporting active processors, Folding@home also determines its computing performance as measured in FLOPS based on the actual execution time of its calculations. Originally this was reported as native FLOPS, that is, the raw performance from each given type of processing hardware. In March 2009 Folding@home began reporting the performance in both native and x86 FLOPS: the latter being an estimation of how many FLOPS the calculation would take on the standard x86 architecture, which is commonly used as a performance reference. Specialized hardware such as GPUs can efficiently perform certain complex functions in a single FLOP which would otherwise require multiple FLOPS on the x86 architecture. This x86 measurement attempts to even out these hardware differences. Despite using conservative conversions, for the GPU and PS3 clients x86 FLOPS are consistently much greater than their native FLOPS and comprise a large majority of Folding@home's FLOP performance.
In 2007 Guinness recognized Folding@home as the most powerful distributed computing network in the world. As of September 13, 2012, the project has 217,215 active CPUs, 19,623 active GPUs, and 16,252 active PS3s, for a total of 3.654 native petaFLOPS (5.354 x86 petaFLOPS). At the same time, the combined efforts of all distributed computing projects under BOINC totals 6.174 petaFLOPS from 447,720 active hosts. Using the Markov state model approach, Folding@home achieves strong scaling across its user base and gains a near-linear speedup for every additional processor. This large and powerful network allows Folding@home to do work not possible any other way.
Active participation in Folding@home has grown steadily since its launch. In March 2002 Google co-founder Sergey Brin launched Google Compute as add-on for the Google Toolbar. Although limited in functionality and scope, it increased participation in Folding@home from 10,000 up to about 30,000 active CPUs. The program ended in October 2005 in favor of the official Folding@home clients, and is no longer available for the Toolbar. Folding@home also gained participants from Genome@home, another distributed computing project from the Pande lab and a sister project to Folding@home. The goal of Genome@home was protein design and associated applications. Following its official conclusion in March 2004, users were asked to donate computing power to Folding@home instead.
Performance
| Date | Folding@home | Fastest supercomputer |
|---|---|---|
| June 2007 | 874 TFLOPS | 280 TFLOP BlueGene/L |
| November 2007 | 1,401 TFLOPS | 478 TFLOP BlueGene/L |
| June 2008 | 1,923 TFLOPS | 1,026 TFLOP Roadrunner |
| November 2008 | 4,021 TFLOPS | 1,105 TFLOP Roadrunner |
| June 2009 | 4,668 native, 8,418 x86 TFLOPS | 1,105 TFLOP Roadrunner |
| November 2009 | Unknown | 1,759 TFLOP Jaguar |
| June 2010 | 3,249 native, 5,674 x86 TFLOPS | 1,759 TFLOP Jaguar |
| November 2010 | 5,729 native, 10,257 x86 TFLOPS | 2,566 TFLOP Tianhe-1A |
| June 2011 | 5,706 native, 9,359 x86 TFLOPS | 8,162 TFLOP K computer |
| November 2011 | 6,012 native, 7,942 x86 TFLOPS | 10,510 TFLOP K computer |
| June 2012 | 4,480 native, 6,544 x86 TFLOPS | 16,325 TFLOP Blue Gene/Q |
On September 16, 2007, due in large part to the participation of Playstation 3s, the Folding@home project officially attained a sustained performance level higher than one native petaFLOP, becoming the first computing system of any kind in the world to do so. On May 7, 2008, the project attained a sustained performance level higher than two native petaFLOPS, followed by the three and four native petaFLOPS milestones on August 20 and September 28, 2008 respectively. It was the first computing project to do so. Then on February 18, 2009, Folding@home achieved a performance level of just above five native petaFLOPS. Most recently, on November 10, 2011, Folding@home's performance exceeded six native petaFLOPS with the equivalent of nearly eight x86 petaFLOPS.
Points
Similarly to other distributed computing projects, Folding@home quantitatively assesses user computing contributions to the project through a credit system. All units from a given protein project have uniform base credit, which is determined by benchmarking one or more Work Units from that project on an official reference machine before the project is released. Each user receives these base points for completing every Work Unit, though through the use of a passkey they can receive additional bonus points for reliably and rapidly completing units which are more computationally demanding or have a greater scientific priority. Users may also receive credit for their work by clients on multiple machines. This generates a fair system of equal pay for equal work, and attempts to align credit with the value of the scientific results.
Users can register their contributions under a team, which combine the points of all their members. A user can start their own team, or they can join an existing team. In some cases, a team may have their own community-driven sources of help or recruitment such as an Internet forum. The points can foster friendly competition between individuals and teams to compute the most for the project, which can benefit the folding community and accelerate scientific research. Individual and team statistics are posted on the Folding@home website.
Software
Folding@home software at the user's end involves three primary components: Work Units, cores, and a client.
Work Units
A Work Unit is the protein data that the client is asked to process. Work Units are a fraction of the simulation between the states in a Markov state model. After the Work Unit has been downloaded and completely processed, it is returned and the respective credit points are awarded, and this cycle then repeats automatically. All Work Units have associated deadlines, and if this deadline is exceeded, the user may not get credit and the unit will be automatically reissued to another participant. As protein folding is serial in nature and many Work Units are generated from their predecessors, this allows the overall simulation process to proceed normally if one is not returned after a certain period of time. Due to these deadlines, the minimum system requirement for Folding@home is a Pentium 3 450 MHz CPU with SSE or newer. However, Work Units for high-performance clients have a much shorter deadline than those for the uniprocessor client, as a major part of the scientific benefit is dependent on rapidly completing simulations.
Before public release, Work Units go through several quality assurance steps to keep problematic ones from becoming fully available. These stages include internal testing, closed beta testing, and open beta testing, before a final full release across all of Folding@home. Folding@home's Work Units are normally processed only once, except in the rare event that errors occur during processing. If this occurs for three different users, the unit is automatically pulled from distribution. The Folding@home support forum can be used to differentiate between problematic hardware and a bad Work Unit.
Cores
Main article: Folding@home coresSpecialized molecular dynamics programs, referred to as "FahCores" and often abbreviated "cores", perform the calculations on the Work Unit behind the scenes. A large majority of Folding@home's cores are based on GROMACS, one of the fastest and most popular molecular dynamics software packages available, which largely consists of manually optimized assembly code and hardware optimizations. Although GROMACS is open-source software and there is a cooperative effort between the Pande lab and GROMACS developers, Folding@home uses a closed-source license for data validity reasons. Less active cores include ProtoMol and SHARPEN. Folding@home has used AMBER, CPMD, Desmond, and TINKER, but these have since been retired and are no longer in active service. Some of these cores perform explicit solvation calculations in which the surrounding solvent (usually water) is modeled atom-by-atom; while others perform implicit solvation methods, where the solvent is treated as a mathematical continuum. The core is separate from the client to enable the scientific methods to be updated automatically without requiring a client update. The cores periodically create calculation checkpoints so that if they are interrupted they can resume work from that point upon startup.
Client
Folding@home participants install a client program on their personal computer or on the PlayStation 3 gaming console. The user interacts with the client, which manages the other software components behind the scenes. Through the client, the user may pause the folding process, open an event log, check the work progress, or view personal statistics. The computer clients run continuously in the background at an extremely low priority, utilizing otherwise unused processing power so that normal computer usage is unaffected. The maximum CPU utilization can also be adjusted through client settings. The client connects to a Folding@home server and retrieves a Work Unit and may also download the appropriate core for the client's settings, operating system, and the underlying hardware architecture. After processing, the Work Unit is returned to the Folding@home servers. Computer clients tailor to uniprocessor and multi-core processors systems, as well as graphics processing units. While these latter clients use significantly more resources, the diversity and power of each hardware architecture provides Folding@home with the ability to efficiently complete many different types of simulations in a timely manner, (in a few weeks or months rather than years) which is of significant scientific value. Together, these clients allow researchers to study biomedical questions previously considered impossible to tackle computationally.
Folding@home software developers put significant work goes into minimizing security issues. For example, clients can be downloaded only from the official Folding@home website or its commercial partners. Folding@home's End-User License Agreement forbids public access to the client source code for security and scientific integrity reasons. Each client will upload and download data only from Stanford's Folding@home data servers (over port 8080, with 80 as an alternative) using 2048-bit digital signatures for verification and will only interact with Folding@home computer files. Thus from a security standpoint it behaves in a similar fashion to a web browser, but is even more secure.
Folding@home's first client was a screensaver, which would run Folding@home while the computer was not otherwise in use. In 2004 the Pande lab collaborated with David Anderson to test a supplemental client on the open-source BOINC framework. This client was released to closed beta in April 2005; however, the approach became unworkable and was abandoned in June 2006. BOINC's fixed architecture limits the types of project it can accommodate and thus was not appropriate for Folding@home.
Graphics processing units
The specialized hardware of GPUs is designed to accelerate rendering of 3D graphics applications such as video games and can significantly outperform CPUs for certain types of calculations. Although limited in generality, this makes GPUs one of the most powerful and rapidly growing computational platforms. As such, general purpose GPU computing is the pursuit of many scientists and researchers. However, GPU hardware is difficult to utilize for non-graphics tasks and usually requires significant algorithm restructuring and an advanced understanding of the underlying architecture. Such customization is challenging, especially to researchers with limited software development resources. Folding@home uses the open source OpenMM library, which uses two API levels to interface molecular simulation software to an underlying hardware architecture. With the addition of hardware optimizations, OpenMM-based GPU simulations do not require significant modification but achieve performance nearly equal to hand-tuned GPU code, and greatly outperform CPU implementations.
Prior to 2010 the computational reliability of GPGPU consumer-grade hardware had remained largely unknown, and circumstantial evidence related to the lack of built-in error detection and correction in GPU memory raised reliability concerns. In the first large-scale test of GPU scientific accuracy, a 2010 study of over 20,000 hosts on the Folding@home network detected soft errors in the memory subsystems of two-thirds of the tested GPUs. These errors strongly correlated to board architecture, though the study concluded that reliable GPU computing was very feasible as long as attention is paid to the hardware characteristics, such as through the use of software-side error detection.
The first generation of Folding@home's Windows GPU client (GPU1) was released to the public on October 2, 2006, delivering a 20-30X speedup for certain calculations over its CPU-based GROMACS counterparts. It was the first time GPUs had been used for either distributed computing or major molecular dynamics calculations. GPU1 gave researchers significant knowledge and experience with the development of GPGPU software, but in response to scientific inaccuracies with DirectX, on April 10, 2008 it was succeeded by GPU2, the second generation of the client. Following its introduction, GPU1 was officially retired on June 6. Compared to GPU1, GPU2 was more scientifically reliable and productive, ran on both ATI and CUDA-enabled Nvidia GPUs, and supported more advanced algorithms, larger proteins, and real-time visualization of the protein simulation. Following this, the third generation of Folding@home's GPU client (GPU3) was released on May 25, 2010. While backwards compatible to GPU2, GPU3 was comparatively more stable and efficient, had greater flexibility in its scientific capabilities, and used OpenMM on top of an OpenCL framework. Although the GPU clients do not natively support the Linux operating system, users with Nvidia graphics cards can run them under the WINE software. GPUs remain Folding@home's most powerful platform in terms of FLOPS; as of September 2012 GPU clients account for 76% of the entire project's x86 FLOP throughput.
PlayStation 3
Further information: Life with PlayStation
Folding@home can also take advantage of the computing power of PlayStation 3's. At the time of its inception and for certain calculations, its main streaming Cell processor delivered a 20x speed increase over PCs, processing power which could not be found on other systems such as the Xbox 360. The PS3's high speed and efficiency introduced other opportunities for worthwhile optimizations, and significantly changed the tradeoff between computational efficiency and overall accuracy, allowing for the utilization of more complex molecular models at little additional computational cost. This allowed Folding@home to run biomedical calculations that would otherwise be computationally infeasible.
The PS3 client was developed in a collaborative effort between Sony and the Pande lab and was first released as a standalone client on March 23, 2007. Its release made Folding@home the first distributed computing project to utilize PS3s. On September 18 of the following year, the PS3 client became a channel of Life with PlayStation on its launch. In terms of the types of calculations it can perform, at the time of its introduction the client took the middle ground between a CPU's flexibility and a GPU's speed. However, unlike CPUs and GPUs, users cannot perform other activities on their PS3 while running Folding@home. The PS3's uniform console environment makes support easier and makes Folding@home more user friendly. The PS3 also has the ability to stream data quickly to its GPU, and is capable of real-time atomic-level visualizations of the current protein dynamics.
Multi-core processing client
Folding@home can also utilize the parallel processing capabilities of modern multi-core processors. The ability to use several CPU cores simultaneously allows completion of the overall folding simulation much faster. Working together, these CPU cores complete single Work Units proportionately faster than the standard uniprocessor client, which reduces the traditional difficulties of scaling a large simulation to many separate processors. While this approach is not only scientifically valuable, the resulting publications would not have been possible without this computing power.
In November 2006 first-generation symmetric multiprocessing (SMP) clients were publicly released for open beta testing, referred to as SMP1. These clients used Message Passing Interface (MPI) communication protocols for parallel processing, as at that time the GROMACS cores were not designed to be used with multiple threads. This was the first time a distributed computing project had utilized MPI, as it had previously been reserved only for supercomputers, and SMP1 represented a landmark in the simulation of protein folding. Although the clients performed well in Unix-based operating systems such as Linux and Mac's OS-X, they were troublesome under Windows. On January 24, 2010, SMP2, the second generation of the SMP clients and the successor to SMP1, was released as an open beta and replaced the complex MPI with a more reliable thread-based implementation.
SMP2 supported a trial of a special category of "bigadv" Work Units, designed for simulating proteins that are unusually large and computationally intensive and have a great scientific priority. These units originally required a minimum of eight CPU cores, which was later increased on February 7, 2012 to sixteen CPU cores. In addition to these additional hardware requirements over standard SMP2 Work Units, they also require more system resources such as RAM and Internet bandwidth. In return, users who run these are rewarded with a 20% increase over SMP2's bonus point system. The bigadv category allows Folding@home to run particularly demanding simulations on long timescales that had previously required the use of supercomputing clusters and could not be performed anywhere else on Folding@home.
V7

The V7 client is the seventh and latest generation of the Folding@home client software, and is a complete rewrite and unification of the previous clients for Microsoft Windows, Mac OS X and Linux operating systems. Like its predecessors, V7 can also run Folding@home in the background at a very low priority, allowing other applications to use CPU resources as they need. It is designed to make the installation, start-up, and operation more user-friendly for novices, as well as offers greater scientific flexibility to researchers than previous clients. V7 uses Trac for managing its bug tickets so that users can see its development process and provide feedback. It was officially released on March 22, 2012.
V7 consists of four integrated elements. The user typically interacts with V7's open-source GUI, known as FAHControl. This has Novice, Advanced, and Expert user interface modes, and has the ability to monitor, configure, and control many remote folding clients from a single computer. FAHControl directs FAHClient – a back-end application that in turn manages each FAHSlot (or "slot"). Each slot acts as replacement for the previously distinct Folding@home v6 uniprocessor, SMP, or GPU computer clients, as it can download, process, and upload Work Units independently. The FAHViewer function, modeled after the PS3's viewer, displays a real-time 3D rendering, if available, of the protein currently being processed.
Comparison to other molecular systems
Rosetta@home is a distributed computing project aimed at protein structure prediction and is one of the most accurate tertiary structure predictors available. The conformational states from Rosetta's software can be used to initialize a Markov state model as starting points for Folding@home simulations. Conversely, structure prediction algorithms can be improved from thermodynamic and kinetic models and the sampling aspects of protein folding simulations. As Rosetta only tries to predict the final folded state, and not how proteins fold, Rosetta@home and Folding@home are complementary and address very different molecular questions.
Anton is a special-purpose supercomputer constructed for molecular dynamics simulations. As of October 2011 Anton and Folding@home are the two most powerful molecular dynamics systems. Anton is unique in its ability to produce single ultra-long computationally expensive molecular trajectories, such as one in 2010 which reached the millisecond range. However, Anton does not use Markov state models for analysis. In 2011 the Pande lab constructed a MSM from two 100-µs Anton simulations and found alternative folding pathways that were not visible through Anton's traditional analysis. They concluded that there was little difference between MSMs constructed from a limited number of long trajectories or one assembled from many shorter trajectories. In June 2011 Folding@home began additional sampling of an Anton simulation in an effort to better determine how its techniques compare to Anton's methods. However, unlike Folding@home's shorter trajectories, which are more amendable to distributed computing and other parallelization techniques, longer trajectories do not require adaptive sampling to sufficiently sample the protein's phase space. Due to this, it is possible that a combination of Anton's and Folding@home's simulation methods would provide a more thorough sampling of this space.
See also
- Computational biology
- Foldit
- List of distributed computing projects
- Molecular dynamics
- Molecular modeling on GPUs
- Rosetta@home
- Software for molecular modeling
- Storage@home
Notes
Note 1: Supercomputer FLOP performance is assessed by running the legacy LINPACK benchmark. This short-term testing has difficulty in accurately reflecting sustained performance on real-world tasks because LINPACK more efficiently maps to supercomputer hardware. Computing systems also vary in architecture and design, so direct comparison is difficult. Despite this, FLOPS remain the primary speed metric used in supercomputing. In contrast, Folding@home determines its FLOPS using wall clock time by measuring how much time its Work Units take to complete.
References
- ^ Pande lab (August 2, 2012). "About Folding@home". Folding@home. Stanford University. Retrieved August 20, 2012.
- Pande lab. "Folding@Home Executive summary" (PDF). Folding@home. Stanford University. Retrieved October 4, 2011.
- ^ Pande lab (2012). "Folding@home homepage". Folding@home. Stanford University. Retrieved August 20, 2012.
- "Folding@home for PlayStation3". Folding@home. Sony. 2008. Retrieved April 5, 2012.
- ^ Pande lab (August 2, 2012). "Folding@home Open Source FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- ^
V. S. Pande, K. Beauchamp, and G. R. Bowman (2010). "Everything you wanted to know about Markov State Models but were afraid to ask". Methods. 52 (1): 99–105. doi:10.1016/j.ymeth.2010.06.002. PMC 2933958. PMID 20570730.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pande lab (July 27, 2012). "Recent Results and Research Papers from Folding@home". Folding@home. Stanford University. Retrieved August 20, 2012.
- ^
Vincent A. Voelz, Gregory R. Bowman, Kyle Beauchamp and Vijay S. Pande (2010). "Molecular simulation of ab initio protein folding for a millisecond folder NTL9(1–39)". Journal of the American Chemical Society. 132 (5): 1526–1528. doi:10.1021/ja9090353. PMC 2835335. PMID 20070076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gregory R. Bowman and Vijay S. Pande (2010). "Protein folded states are kinetic hubs". Proceedings of the National Academy of Sciences. 107 (24): 10890. Bibcode:2010PNAS..10710890B. doi:10.1073/pnas.1003962107.
- Fabrizio Marinelli, Fabio Pietrucci, Alessandro Laio, Stefano Piana (2009). Pande, Vijay S (ed.). "A Kinetic Model of Trp-Cage Folding from Multiple Biased Molecular Dynamics Simulations". PLoS Computational Biology. 8 (5): e1000452. doi:10.1371/journal.pcbi.1000452.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - "So Much More to Know". Science. 309 (5731): 78–102. 2005. doi:10.1126/science.309.5731.78b. PMID 15994524.
- ^
Heath Ecroyd, John A. Carver (2008). "Unraveling the mysteries of protein folding and misfolding". IUBMB Life. 60 (12): 769–774. doi:10.1002/iub.117. PMID 18767168.
{{cite journal}}:|format=requires|url=(help) - ^
Yiwen Chen, Feng Ding, Huifen Nie, Adrian W. Serohijos, Shantanu Sharma, Kyle C. Wilcox, Shuangye Yin, Nikolay V. Dokholyan (2008). "Protein folding: Then and now". Archives of Biochemistry and Biophysics. 469 (1): 4–19. doi:10.1016/j.abb.2007.05.014. PMC 2173875. PMID 17585870.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Leila M Luheshi, Damian Crowther, Christopher Dobson (2008). "Protein misfolding and disease: from the test tube to the organism". Current Opinion in Chemical Biology. 12 (1): 25–31. doi:10.1016/j.cbpa.2008.02.011. PMID 18295611.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
C. D. Snow, E. J. Sorin, Y. M. Rhee, and V. S. Pande. (2005). "How well can simulation predict protein folding kinetics and thermodynamics?". Annual Reviews of Biophysics. 34: 43–69. doi:10.1146/annurev.biophys.34.040204.144447. PMID 15869383.
{{cite journal}}:|format=requires|url=(help)CS1 maint: multiple names: authors list (link) - A. Verma, S.M. Gopal, A. Schug, J.S. Oh, K.V. Klenin, K.H. Lee, and W. Wenzel (2008). "Massively Parallel All Atom Protein Folding in a Single Day". Advances in Parallel Computing. 15: 527–534. ISBN 978-1-58603-796-3. ISSN 0927-5452.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Vijay S. Pande, Ian Baker, Jarrod Chapman, Sidney P. Elmer, Siraj Khaliq, Stefan M. Larson, Young Min Rhee, Michael R. Shirts, Christopher D. Snow, Eric J. Sorin, Bojan Zagrovic (2002). "Atomistic protein folding simulations on the submillisecond timescale using worldwide distributed computing". Biopolymers. 68 (1): 91–109. doi:10.1002/bip.10219. PMID 12579582.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
G. Bowman, V. Volez, and V. S. Pande (2011). "Taming the complexity of protein folding". Current Opinion in Structural Biology. 21 (1): 4–11. doi:10.1016/j.sbi.2010.10.006. PMC 3042729. PMID 21081274.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Robert B Best (2012). "Atomistic molecular simulations of protein folding". Current Opinion in Structural Biology. 22 (1): 52–61. doi:10.1016/j.sbi.2011.12.001. PMID 22257762.
{{cite journal}}:|format=requires|url=(help) - ^
TJ Lane, Gregory Bowman, Robert McGibbon, Christian Schwantes, Vijay Pande, and Bruce Borden (September 10, 2012). "Folding@home Simulation FAQ". Folding@home. Stanford University. Retrieved September 10, 2012.
{{cite web}}: CS1 maint: multiple names: authors list (link) -
Gregory R. Bowman, Daniel L. Ensign, and Vijay S. Pande (2010). "Enhanced Modeling via Network Theory: Adaptive Sampling of Markov State Models". Journal of Chemical Theory and Computation. 6 (3): 787–794. doi:10.1021/ct900620b.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Vijay Pande (June 8, 2012). "FAHcon 2012: Thinking about how far FAH has come". Folding@home. typepad.com. Retrieved June 12, 2012.
- Christopher D. Snow, Houbi Ngyen, Vijay S. Pande, and Martin Gruebele (2002). "Absolute comparison of simulated and experimental protein-folding dynamics" (PDF). Nature. 420 (6911): 102–106. Bibcode:2002Natur.420..102S. doi:10.1038/nature01160. PMID 12422224.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kyle A. Beauchamp, Daniel L. Ensign, Rhiju Das, and Vijay S. Pande (2011). "Quantitative comparison of villin headpiece subdomain simulations and triplet–triplet energy transfer experiments". Proceedings of the National Academy of Sciences. 108 (31): 12734. Bibcode:2011PNAS..10812734B. doi:10.1073/pnas.1010880108.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Greg Bowman awarded the 2010 Kuhn Paradigm Shift Award". simtk.org. SimTK: MSMBuilder. March 29, 2010. Retrieved April 5, 2012.
- "Biophysical Society Names Five 2012 Award Recipients". Biophysics.org. Biophysical Society. August 17, 2011. Retrieved April 5, 2012.
- "Folding@home – Awards". Folding@home. Stanford University. June 2006. Retrieved August 31, 2012.
- Antonella De Jaco, Michael Z. Lin, Noga Dubi, Davide Comoletti, Meghan T. Miller, Shelley Camp, Mark Ellisman, Margaret T. Butko, Roger Y. Tsien, and Palmer Taylor (2010). "Neuroligin Trafficking Deficiencies Arising from Mutations in the a/ß-Hydrolase Fold Protein Family". Journal of Biological Chemistry. 285 (37): 28674–28682. doi:10.1074/jbc.M110.139519. PMC 2937894. PMID 20615874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Vittorio Bellotti and Monica Stoppini (2009). "Protein Misfolding Diseases" (PDF). The Open Biology Journal. 2: 228–234.
- ^ Pande lab (May 30, 2012). "Folding@home Diseases Studied FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Fred E. Cohen & Jeffery W. Kelly (2003). "Therapeutic approaches to protein misfolding diseases". Nature. 426 (6968): 905–9. doi:10.1038/nature02265. PMID 14685252.
{{cite journal}}:|format=requires|url=(help) - ^
Collier, Leslie; Balows, Albert; Sussman, Max (1998). Mahy, Brian; Collier, Leslie (eds.). Topley and Wilson's Microbiology and Microbial Infections. Vol. 1, Virology (ninth ed.). London: Arnold. pp. 75–91. ISBN 978-0-340-66316-5.
{{cite book}}: templatestyles stripmarker in|volume=at position 2 (help) - ^
Chun Song, Shen Lim, Joo Tong (2009). "Recent advances in computer-aided drug design". Briefings in Bioinformatics. 10 (5): 579–91. doi:10.1093/bib/bbp023. PMID 19433475.
{{cite journal}}:|format=requires|url=(help)CS1 maint: multiple names: authors list (link) - ^ Pande lab (2012). "Folding@Home Press FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Christian "schwancr" Schwantes (Pande lab member) (August 15, 2011). "Projects 7808 and 7809 to full fah". Folding@home. phpBB Group. Retrieved October 16, 2011.
- Del Lucent, V. Vishal, and Vijay S. Pande (2007). "Protein folding under confinement: A role for solvent". Proceedings of the National Academy of Sciences of the United States of America. 104 (25): 10430–10434. Bibcode:2007PNAS..10410430L. doi:10.1073/pnas.0608256104.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Vincent A. Voelz, Vijay R. Singh, William J. Wedemeyer, Lisa J. Lapidus, and Vijay S. Pande (2010). "Unfolded-State Dynamics and Structure of Protein L Characterized by Simulation and Experiment". Journal of the American Chemical Society. 132 (13): 4702–4709. doi:10.1021/ja908369h. PMC 2853762. PMID 20218718.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pande lab (August 18, 2011). "Folding@home Main FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- ^ Vijay Pande (April 23, 2008). "Folding@home and Simbios". Folding@home. typepad.com. Retrieved November 9, 2011.
- ^ Vijay Pande (October 25, 2011). "Re: Suggested Changes to F@h Website". Folding@home. phpBB Group. Retrieved October 25, 2011.
- ^ Caroline Hadley (2004). "Biologists think bigger". EMBO Reports. 12 (5): 236–238. doi:10.1038/sj.embor.7400108.
- S. Pronk, P. Larsson, I. Pouya, G.R. Bowman, I.S. Haque, K. Beauchamp, B. Hess, V.S. Pande, P.M. Kasson, E. Lindahl (2011). "Copernicus: A new paradigm for parallel adaptive molecular dynamics". 2011 International Conference for High Performance Computing, Networking, Storage and Analysis: 1–10, 12–18.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - G Brent Irvine, Omar M El-Agnaf, Ganesh M Shankar, and Dominic M Walsh (2008). "Protein Aggregation in the Brain: The Molecular Basis for Alzheimer's and Parkinson's Diseases" (review). Molecular Medicine. 14 (7–8): 451–464. doi:10.2119/2007-00100.Irvine. PMC 2274891. PMID 18368143.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Claudio Soto, Lisbell D. Estrada (2008). "Protein Misfolding and Neurodegeneration". Archives of Neurology. 65 (2): 184–189. doi:10.1001/archneurol.2007.56. PMID 18268186.
{{cite journal}}:|format=requires|url=(help) -
Robin Roychaudhuri, Mingfeng Yang, Minako M. Hoshi and David B. Teplow (2008). "Amyloid β-Protein Assembly and Alzheimer Disease". Journal of Biological Chemistry. 284 (8): 4749–53. doi:10.1074/jbc.R800036200. PMID 18845536.
{{cite journal}}:|format=requires|url=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^
Nicholas W. Kelley, V. Vishal, Grant A. Krafft, and Vijay S. Pande. (2008). "Simulating oligomerization at experimental concentrations and long timescales: A Markov state model approach". Journal of Chemical Physics. 129 (21): 214707. Bibcode:2008JChPh.129u4707K. doi:10.1063/1.3010881. PMC 2674793. PMID 19063575.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
P. Novick, J. Rajadas, C.W. Liu, N. W. Kelley, M. Inayathullah, and V. S. Pande (2011). Buehler, Markus J. (ed.). "Rationally Designed Turn Promoting Mutation in the Amyloid-β Peptide Sequence Stabilizes Oligomers in Solution". PLoS ONE. 6 (7): e21776. doi:10.1371/journal.pone.0021776. PMC 3142112. PMID 21799748.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Aabgeena Naeem and Naveed Ahmad Fazili (2011). "Defective Protein Folding and Aggregation as the Basis of Neurodegenerative Diseases: The Darker Aspect of Proteins". Cell Biochemistry and Biophysics. 61 (2): 237–50. doi:10.1007/s12013-011-9200-x. PMID 21573992.
{{cite journal}}:|format=requires|url=(help) - ^
Gregory R Bowman, Xuhui Huang, and Vijay S Pande (2010). "Network models for molecular kinetics and their initial applications to human health". Cell Research. 20 (6): 622–630. doi:10.1038/cr.2010.57. PMID 20421891.
{{cite journal}}:|format=requires|url=(help)CS1 maint: multiple names: authors list (link) - Vijay Pande (December 18, 2008). "New FAH results on possible new Alzheimer's drug presented". Folding@home. typepad.com. Retrieved September 23, 2011.
- Paul A. Novick, Dahabada H. Lopes, Kim M. Branson, Alexandra Esteras-Chopo, Isabella A. Graef, Gal Bitan, and Vijay S. Pande (2012). "Design of β-Amyloid Aggregation Inhibitors from a Predicted Structural Motif". Journal of Medicinal Chemistry. 55 (7): 3002–10. doi:10.1021/jm201332p. PMID 22420626.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - yslin (Pande lab member) (July 22, 2011). "New project p6871 [Classic]". Folding@home. phpBB Group. Retrieved March 17, 2012.(registration required)
- Pande lab. "Project 6871 Description". Folding@home. Stanford University. Retrieved September 27, 2011.
- Walker FO (2007). "Huntington's disease". Lancet. 369 (9557): 220. doi:10.1016/S0140-6736(07)60111-1. PMID 17240289.
{{cite journal}}: More than one of|pages=and|page=specified (help) -
Nicholas W. Kelley, Xuhui Huang, Stephen Tam, Christoph Spiess, Judith Frydman and Vijay S. Pande (2009). "The predicted structure of the headpiece of the Huntingtin protein and its implications on Huntingtin aggregation". Journal of Molecular Biology. 388 (5): 919–27. doi:10.1016/j.jmb.2009.01.032. PMC 2677131. PMID 19361448.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Susan W Liebman & Stephen C Meredith (2010). "Protein folding: Sticky N17 speeds huntingtin pile-up". Nature — Chemical Biology. 6 (1): 7–8. doi:10.1038/nchembio.279. PMID 20016493.
- Diwakar Shukla (Pande lab member) (February 10, 2012). "Project 8021 released to beta". Folding@home. phpBB Group. Retrieved March 17, 2012.(registration required)
- M Hollstein, D Sidransky, B Vogelstein and CC Harris (1991). "p53 mutations in human cancers". Science. 253 (5015): 49–53. Bibcode:1991Sci...253...49H. doi:10.1126/science.1905840. PMID 1905840.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - L. T. Chong, C. D. Snow, Y. M. Rhee, and V. S. Pande. (2004). "Dimerization of the p53 Oligomerization Domain: Identification of a Folding Nucleus by Molecular Dynamics Simulations". Journal of Molecular Biology. 345 (4): 869–878. doi:10.1016/j.jmb.2004.10.083. PMID 15588832.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - mah3, Vijay Pande (September 24, 2004). "F@H project publishes results of cancer related research". MaximumPC.com. Future US, Inc. Retrieved April 5, 2012.
{{cite web}}: CS1 maint: numeric names: authors list (link) To our knowledge, this is the first peer-reviewed results from a distributed computing project related to cancer. - Lillian T. Chong, William C. Swope, Jed W. Pitera, and Vijay S. Pande (2005). "Kinetic Computational Alanine Scanning: Application to p53 Oligomerization". Journal of Molecular Biology. 357 (3): 1039–1049. doi:10.1016/j.jmb.2005.12.083. PMID 16457841.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Almeida MB, do Nascimento JL, Herculano AM, and Crespo-López ME (2011). "Molecular chaperones: toward new therapeutic tools". Journal of Molecular Biology. 65 (4): 239–43. doi:10.1016/j.biopha.2011.04.025. PMID 21737228.
{{cite journal}}:|format=requires|url=(help)CS1 maint: multiple names: authors list (link) - Vijay Pande (September 28, 2007). "Nanomedicine center". Folding@home. typepad.com. Retrieved September 23, 2011.
- Vijay Pande (December 22, 2009). "Release of new Protomol (Core B4) WUs". Folding@home. typepad.com. Retrieved September 23, 2011.
- Pande lab. "Project 180 Description". Folding@home. Stanford University. Retrieved September 27, 2011.
- TJ Lane (Pande lab member) (June 8, 2011). "Project 7600 in Beta". Folding@home. phpBB Group. Retrieved September 27, 2011.(registration required)
- TJ Lane (Pande lab member) (June 8, 2011). "Project 7600 Description". Folding@home. Stanford University. Retrieved March 31, 2012.
- "Scientists boost potency, reduce side effects of IL-2 protein used to treat cancer". MedicalXpress.com. Medical Xpress. March 18, 2012. Retrieved April 5, 2012.
- Aron M. Levin, Darren L. Bates, Aaron M. Ring, Carsten Krieg, Jack T. Lin, Leon Su, Ignacio Moraga, Miro E. Raeber, Gregory R. Bowman, Paul Novick, Vijay S. Pande, C. Garrison Fathman, Onur Boyman, and K. Christopher Garcia (2012). "Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'". Nature. 484 (7395): 529–33. Bibcode:2012Natur.484..529L. doi:10.1038/nature10975. PMC 3338870. PMID 22446627.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rauch F, Glorieux FH (2004). "Osteogenesis imperfecta". Lancet. 363 (9418): 1377–85. doi:10.1016/S0140-6736(04)16051-0. PMID 15110498.
- Fratzl, Peter (2008). Collagen: structure and mechanics. ISBN 978-0-387-73905-2. Retrieved March 17, 2012.
- Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ (2009). "Molecular and mesoscale disease mechanisms of Osteogenesis Imperfecta". Biophysical Journal. 97 (3): 857–865. Bibcode:2009BpJ....97..857G. doi:10.1016/j.bpj.2009.04.059. PMC 2718154. PMID 19651044.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sanghyun Park, Randall J. Radmer, Teri E. Klein, and Vijay S. Pande (2005). "A New Set of Molecular Mechanics Parameters for Hydroxyproline and Its Use in Molecular Dynamics Simulations of Collagen-Like Peptides". Journal of Computational Chemistry. 26 (15): 1612–1616. doi:10.1002/jcc.20301. PMID 16170799.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Knobler S, Mack A, Mahmoud A, Lemon S (ed.). "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, D.C.: The National Academies Press. pp. 60–61.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: editors list (link) - Potter, CW (2006). "A History of Influenza". J Appl Microbiol. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290.
{{cite journal}}: Unknown parameter|month=ignored (help)(subscription required) - Hana Robson Marsden, Itsuro Tomatsu and Alexander Kros (2011). "Model systems for membrane fusion". Chemical Society Reviews. 40 (3): 1572–1585. doi:10.1039/c0cs00115e. PMID 21152599.
{{cite journal}}:|format=requires|url=(help) - Peter Kasson. "Membrane Fusion". Kasson lab. Stanford University. Retrieved April 5, 2012.
- Peter M. Kasson, Afra Zomorodian, Sanghyun Park, Nina Singhal, Leonidas J. Guibas and Vijay S. Pande (2007). "Persistent voids: a new structural metric for membrane fusion". Bioinformatics. 23 (14): 1753–1759. doi:10.1093/bioinformatics/btm250. PMID 17488753.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Peter M. Kasson, Daniel L. Ensign and Vijay S. Pande (2009). "Combining Molecular Dynamics with Bayesian Analysis To Predict and Evaluate Ligand-Binding Mutations in Influenza Hemagglutinin". Journal of the American Chemical Society. 131 (32): 11338–11340. doi:10.1021/ja904557w. PMC 2737089. PMID 19637916.
- Peter M. Kasson, Vijay S. Pande (2009). "Combining mutual information with structural analysis to screen for functionally important residues in influenza hemagglutinin". Pacific Symposium on Biocomputing: 492–503. PMC 2811693. PMID 19209725.
- Gregory Bowman (Pande lab Member). "Project 10125". Folding@home. phpBB Group. Retrieved December 2, 2011.(registration required)
- Vijay Pande (February 24, 2012). "Protein folding and viral infection". Folding@home. typepad.com. Retrieved March 4, 2012.
- Vijay Pande (February 27, 2012). "New methods for computational drug design". Folding@home. typepad.com. Retrieved April 1, 2012.
-
Guha Jayachandran, M. R. Shirts, S. Park, and V. S. Pande (2006). "Parallelized-Over-Parts Computation of Absolute Binding Free Energy with Docking and Molecular Dynamics". Journal of Chemical Physics. 125 (8): 084901. Bibcode:2006JChPh.125h4901J. doi:10.1063/1.2221680. PMID 16965051.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pande lab. "Project 10721 Description". Folding@home. Stanford University. Retrieved September 27, 2011.
- ^ Gregory Bowman (July 23, 2012). "Searching for new drug targets". Folding@home. typepad.com. Retrieved September 27, 2011.
- Gregory R. Bowman and Phillip L. Geissler (2012). "Equilibrium fluctuations of a single folded protein reveal a multitude of potential cryptic allosteric sites". PNAS. 109 (29): 11681. Bibcode:2012PNAS..10911681B. doi:10.1073/pnas.1209309109.
{{cite journal}}: Unknown parameter|month=ignored (help) - Paula M. Petrone, Christopher D. Snow, Del Lucent, and Vijay S. Pande (2008). "Side-chain recognition and gating in the ribosome exit tunnel". Proceedings of the National Academy of Sciences. 105 (43): 16549. Bibcode:2008PNAS..10516549P. doi:10.1073/pnas.0801795105.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pande lab. "Project 5765 Description". Folding@home. Stanford University. Retrieved December 2, 2011.
- ^ Pande lab (April 4, 2009). "Folding@home FLOP FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Vijay Pande (March 18, 2009). "FLOPS". Folding@home. typepad.com. Retrieved October 11, 2011.
- ^
Pande lab (updated daily). "Client Statistics by OS". Folding@home. Stanford University. Retrieved September 13, 2012.
{{cite web}}: Check date values in:|date=(help) - Pande lab (May 30, 2012). "PS3 FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- "Most powerful distributed computing network". Guinnessworldrecords.com. Guinness World Records. September 16, 2007. Retrieved April 6, 2012.
- "BOINC Combined Credit Overview". BOINCstats.com. BOINC Stats. Retrieved June 12, 2012.
- Vijay Pande (October 21, 2007). "Fun fact: FAH growth over time". Folding@home. typepad.com. Retrieved October 21, 2011.
- Pande lab. "Active CPUs" (Image). Folding@home. Stanford University. Retrieved August 30, 2011.
- Shankland, Stephen (March 22, 2002). "Google takes on supercomputing". CNet News.
- ^ "Futures in Biotech 27: Folding@home at 1.3 Petaflops" (Interview, webcast). Castroller.com. CastRoller. December 28, 2007. Retrieved April 5, 2012.
- Google (2007). "Your computer's idle time is too precious to waste". Archived from the original on June 11, 2008. Retrieved August 31, 2012.
{{cite news}}:|author=has generic name (help) - Vijay Pande, Stefan Larson (March 4, 2002). "Genome@home Updates". April 15, 2004 Update. Retrieved March 17, 2012.
- 7im (May 17, 2012). "Re: Overall F@H Stats Graph?". Folding@home. phpBB Group. Retrieved August 24, 2012.
{{cite web}}: CS1 maint: numeric names: authors list (link) - "TOP500 List — June 2007". top500.org. Top500. June 2007. Retrieved August 12, 2012.
- "Client Stats by OS" (Image). imageshack. November 19, 2007. Retrieved August 23, 2012.
- "TOP500 List — November 2007". top500.org. Top500. November 2007. Retrieved August 12, 2012.
- "Re:Highwater Mark". phpBB Group. June 19, 2008. Retrieved August 24, 2012.
- "TOP500 List — June 2008". top500.org. Top500. June 2008. Retrieved August 12, 2012.
- ^ "Increase in 'active' PS3 folders pushes Folding@home past 4 Petaflops!". team52735.blogspot.com. Blogspot. September 29, 2008. Retrieved August 23, 2012.
- "TOP500 List — November 2008". top500.org. Top500. November 2008. Retrieved August 12, 2012.
- "Google Translate – Distributed Computing Forum in Chinese". Google. June 1, 2009. Retrieved August 24, 2012.
- "TOP500 List — June 2009". top500.org. Top500. June 2009. Retrieved August 12, 2012.
- "TOP500 List — November 2009". top500.org. Top500. November 2009. Retrieved August 12, 2012.
- ^
screen317, others. "Folding@home Stats". Google. Retrieved August 23, 2012.
{{cite web}}: CS1 maint: numeric names: authors list (link) - "TOP500 List — June 2010". top500.org. Top500. June 2010. Retrieved August 12, 2012.
- "TOP500 List — November 2010". top500.org. Top500. November 2010. Retrieved August 12, 2012.
- flight8848 (June 9, 2011). "星空下闪耀的双子星—影驰GTX560黑将试用Ⅴ. 通用运算及总结 – 玩家堂官方硬件团购及活动区 – 玩家堂论坛-硬件爱好者和电脑玩家的天堂" (in Chinese). itocp.com. Retrieved September 16, 2012.
{{cite web}}: CS1 maint: numeric names: authors list (link) - "TOP500 List — June 2011". top500.org. Top500. June 2011. Retrieved August 12, 2012.
- ^ Jesse Victors (November 10, 2011). "Six Native PetaFLOPS". Folding@home. phpBB Group. Retrieved November 11, 2011.
- "TOP500 List — November 2011". top500.org. Top500. November 2011. Retrieved August 12, 2012.
- "TOP500 List — June 2012". top500.org. Top500. June 2012. Retrieved August 12, 2012.
- Vijay Pande (September 16, 2007). "Crossing the petaFLOPS barrier". Folding@home. typepad.com. Retrieved August 28, 2011.
- PlayStation Press Release (September 10, 2007). "PLAYSTATION®3 Helps Folding@Home Become the First Distributed Computing Network to Reach the Petaflop Milestone". us.playstation.com. Sony Computer Entertainment America LLC. Retrieved April 5, 2012.
- "Folding@Home reach 2 Petaflops". n4g.com. HAVAmedia. May 8, 2008. Retrieved April 5, 2012.
- "NVIDIA Achieves Monumental Folding@Home Milestone With Cuda". nvidia.com. NVIDIA Corporation. August 26, 2008. Retrieved April 5, 2012.
- "3 PetaFLOP barrier". longecity.org. Longecity. August 19, 2008. Retrieved April 5, 2012.
- Dragan Zakic (May 2009). "Community Grid Computing — Studies in Parallel and Distributed Systems" (PDF). Massey University College of Sciences. Massey University. Retrieved April 5, 2012.
- Vijay Pande (February 18, 2009). "Folding@home Passes the 5 petaFLOP Mark". Folding@home. typepad.com. Retrieved August 31, 2011.
- "Crossing the 5 petaFLOPS barrier". longecity.org. Longecity. February 18, 2009. Retrieved April 5, 2012.
- ^ Pande lab (August 20, 2012). "Folding@home Points FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- ^ Pande lab (August 16, 2012). "Folding@home Points FAQ (New Benchmark Machine – January 2010)" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Pande lab (July 23, 2012). "Folding@home Passkey FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- ^ Peter Kasson (Pande lab member) (January 24, 2010). "upcoming release of SMP2 cores". Folding@home. phpBB Group. Retrieved September 30, 2011.
- "Official Extreme Overclocking Folding@home Team Forum". forums.extremeoverclocking.com. Extreme Overclocking. Retrieved April 5, 2012.
- ^
Adam Beberg, Daniel Ensign, Guha Jayachandran, Siraj Khaliq, Vijay Pande (2009). "Folding@home: Lessons From Eight Years of Volunteer Distributed Computing" (PDF). Parallel & Distributed Processing, IEEE International Symposium: 1–8. doi:10.1109/IPDPS.2009.5160922. ISBN 978-1-4244-3751-1. ISSN 1530-2075.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Norman Chan (April 6, 2009). "Help Maximum PC's Folding Team Win the Next Chimp Challenge!". Maximumpc.com. Future US, Inc. Retrieved September 6, 2011.
- ^ Pande lab (June 11, 2012). "Folding@home SMP FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Vijay Pande (April 5, 2011). "More transparency in testing". Folding@home. typepad.com. Retrieved October 14, 2011.
- Bruce Borden (August 7, 2011). "Re: Gromacs Cannot Continue Further". Folding@home. phpBB Group. Retrieved August 7, 2011.
- PantherX (October 1, 2011). "Re: Project 6803: (Run 4, Clone 66, Gen 255)". Folding@home. phpBB Group. Retrieved October 9, 2011.
- PantherX (October 31, 2010). "Troubleshooting Bad WUs". Folding@home. phpBB Group. Retrieved August 7, 2011.
- Carsten Kutzner, David Van Der Spoel, Martin Fechner, Erik Lindahl, Udo W. Schmitt, Bert L. De Groot, and Helmut Grubmüller (2007). "Speeding up parallel GROMACS on high-latency networks". Journal of Computational Chemistry. 28 (12): 2075–2084. doi:10.1002/jcc.20703. PMID 17405124.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Berk Hess, Carsten Kutzner, David van der Spoel, and Erik Lindahl (2008). "GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation". Journal of Chemical Theory and Computation. 4 (3): 435–447. doi:10.1021/ct700301q.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pande lab (August 19, 2012). "Folding@home Gromacs FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Pande lab (August 7, 2012). "Folding@home Frequently Asked Questions (FAQ) Index" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- Vijay Pande (September 25, 2009). "Update on new FAH cores and clients". Folding@home. typepad.com. Retrieved February 24, 2012.
- ^
M. S. Friedrichs, P. Eastman, V. Vaidyanathan, M. Houston, S. LeGrand, A. L. Beberg, D. L. Ensign, C. M. Bruns, V. S. Pande (2009). "Accelerating Molecular Dynamic Simulation on Graphics Processing Units". Journal of Computational Chemistry. 30 (6): 864–72. doi:10.1002/jcc.21209. PMC 2724265. PMID 19191337.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pande lab (August 19, 2012). "Folding@home Petaflop Initiative". Folding@home. Stanford University. Retrieved August 20, 2012.
- ^ Pande lab (February 10, 2011). "Windows Uniprocessor Client Installation Guide". Folding@home. Stanford University. Retrieved August 20, 2012.
- PantherX (September 2, 2010). "Re: Can Folding@home damage any part of my PC?". Folding@home. phpBB Group. Retrieved February 25, 2012.
- ^ Pande lab. "Folding@home Distributed Computing Client". Folding@home. Stanford University. Retrieved August 20, 2012.
- Vijay Pande (June 28, 2008). "Folding@home's End User License Agreement (EULA)". Folding@home. Retrieved May 15, 2012.
- ^ Pande lab (May 30, 2012). "Uninstalling Folding@home FAQ". Folding@home. Stanford University. Retrieved August 20, 2012.
- M. R. Shirts and V. S. Pande. (2000). "Screen Savers of the World, Unite!". Science. 290 (5498): 1903–1904. doi:10.1126/science.290.5498.1903. PMID 17742054.
- Rattledagger, Vijay Pande (April 1, 2005). "Folding@home client for BOINC in beta "soon"". Boarddigger.com. Anandtech.com. Retrieved April 5, 2012.
- ^ Pande lab (May 30, 2012). "High Performance FAQ" (FAQ). Folding@home. Stanford University. Retrieved August 20, 2012.
- John D. Owens, David Luebke, Naga Govindaraju, Mark Harris, Jens Krüger, Aaron Lefohn, Timothy J. Purcell (2007). "A Survey of General-Purpose Computation on Graphics Hardware". Computer Graphics Forum. 26 (1): 80–113. doi:10.1111/j.1467-8659.2007.01012.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - P. Eastman and V. S. Pande (2010). "OpenMM: A Hardware-Independent Framework for Molecular Simulations". Computing in Science and Engineering. 12 (4): 34–39. doi:10.1109/MCSE.2010.27. ISSN 1521-9615.
- I. Haque and V. S. Pande (2010). "Hard Data on Soft Errors: A Large-Scale Assessment of Real-World Error Rates in GPGPU". 2010 10th IEEE/ACM International Conference on Cluster, Cloud and Grid Computing (CCGrid): 691–696. doi:10.1109/CCGRID.2010.84. ISBN 978-1-4244-6987-1.
- ^ Pande lab (March 18, 2011). "ATI FAQ" (FAQ). Folding@home. Stanford University. Retrieved March 21, 2012.
- Vijay Pande (May 23, 2008). "GPU news (about GPU1, GPU2, & NVIDIA support)". Folding@home. typepad.com. Retrieved September 8, 2011.
- Travis Desell, Anthony Waters, Malik Magdon-Ismail, Boleslaw K. Szymanski, Carlos A. Varela, Matthew Newby, Heidi Newberg, Andreas Przystawik, and David Anderson (2009). "Accelerating the MilkyWay@Home volunteer computing project with GPUs". 8th International Conference on Parallel Processing and Applied Mathematics (PPAM 2009) Part I. pp. 276–288. ISBN 978-3-642-14389-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Vijay Pande (April 10, 2008). "GPU2 open beta". Folding@home. typepad.com. Retrieved September 7, 2011.
- Vijay Pande (April 15, 2008). "Updates to the Download page/GPU2 goes live". Folding@home. typepad.com. Retrieved September 7, 2011.
- Vijay Pande (April 11, 2008). "GPU2 open beta going well". Folding@home. typepad.com. Retrieved September 7, 2011.
- ^ Vijay Pande (April 24, 2010). "Prepping for the GPU3 rolling: new client and NVIDIA FAH GPU clients will (in the future) need CUDA 2.2 or later". Folding@home. typepad.com. Retrieved September 8, 2011.
- Vijay Pande (May 25, 2010). "Folding@home: Open beta release of the GPU3 client/core". Folding@home. typepad.com. Retrieved September 7, 2011.
- Joseph Coffland (Pande lab member) (October 13, 2011). "Re: FAHClient V7.1.38 released (4th Open-Beta)". Folding@home. phpBB Group. Retrieved October 15, 2011.
- "NVIDIA GPU3 Linux/Wine Headless Install Guide". Folding@home. phpBB Group. November 8, 2008. Retrieved September 5, 2011.
- ^
Edgar Luttmann, Daniel L. Ensign, Vishal Vaidyanathan, Mike Houston, Noam Rimon, Jeppe Øland, Guha Jayachandran, Mark Friedrichs, Vijay S. Pande (2008). "Accelerating Molecular Dynamic Simulation on the Cell processor and PlayStation 3". Journal of Computational Chemistry. 30 (2): 268–274. doi:10.1002/jcc.21054. PMID 18615421.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ David E. Williams (October 20, 2006). "PlayStation's serious side: Fighting disease". CNN. Retrieved April 5, 2012.
- Jerry Liao (March 23, 2007). "The Home Cure: PlayStation 3 to Help Study Causes of Cancer". mb.com. Manila Bulletin Publishing Corporation. Retrieved April 5, 2012.
- Lou Kesten, Associated Press (March 26, 2007). "Week in video-game news: 'God of War II' storms the PS2; a PS3 research project". Post-Gazette.com. Pittsburgh Post-Gazette. Retrieved April 5, 2012.
- Elaine Chow (September 18, 2008). "PS3 News Service, Life With Playstation, Now Up For Download". Gizmodo.com. Gizmodo. Retrieved April 5, 2012.
- Vijay Pande (September 18, 2008). "Life with Playstation – a new update to the FAH/PS3 client". Folding@home. typepad.com. Retrieved February 24, 2012.
- ^ Vijay Pande (June 15, 2008). "What does the SMP core do?". Folding@home. typepad.com. Retrieved September 7, 2011.
- ^ Vijay Pande (March 8, 2008). "New Windows client/core development (SMP and classic clients)". Folding@home. typepad.com. Retrieved September 30, 2011.
- Vijay Pande (June 17, 2009). "How does FAH code development and sysadmin get done?". Folding@home. typepad.com. Retrieved October 14, 2011.
- ^ Peter Kasson (Pande lab member) (July 15, 2009). "new release: extra-large work units". Folding@home. phpBB Group. Retrieved October 9, 2011.
- Vijay Pande (February 7, 2012). "Update on "bigadv-16", the new bigadv rollout". Folding@home. typepad.com. Retrieved February 9, 2012.
- Vijay Pande (July 2, 2011). "Change in the points system for bigadv work units". Folding@home. typepad.com. Retrieved February 24, 2012.
- ^ Pande lab (March 23, 2012). "Windows (FAH V7) Install Guide". Folding@home. Stanford University. Retrieved March 21, 2012.
- ^ Vijay Pande (March 29, 2011). "Client version 7 now in open beta". Folding@home. typepad.com. Retrieved August 14, 2011.
- Vijay Pande (March 31, 2011). "Core 16 for ATI released; also note on NVIDIA GPU support for older boards". Folding@home. typepad.com. Retrieved September 7, 2011.
- Vijay Pande (March 22, 2012). "Web page revamp and v7 rollout". Folding@home. typepad.com. Retrieved March 22, 2012.
- Lensink MF, Méndez R, Wodak SJ (2007). "Docking and scoring protein complexes: CAPRI 3rd Edition". Proteins. 69 (4): 704–18. doi:10.1002/prot.21804. PMID 17918726.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gregory R. Bowman and Vijay S. Pande (2009). "Simulated tempering yields insight into the low-resolution Rosetta scoring function". Proteins: Structure, Function, and Bioinformatics. 74 (3): 777–88. doi:10.1002/prot.22210. PMID 18767152.
- G. R. Bowman and V. S. Pande (2009). Hofmann, Andreas (ed.). "The Roles of Entropy and Kinetics in Structure Prediction". PLoS ONE. 4 (6): e5840. Bibcode:2009PLoSO...4.5840B. doi:10.1371/journal.pone.0005840. PMC 2688754. PMID 19513117.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Gen_X_Accord, Vijay Pande (June 11, 2006). "Folding@home vs. Rosetta@home". Rosetta@home forums. University of Washington. Retrieved April 6, 2012.
- Vijay Pande (October 13, 2011). "Comparison between FAH and Anton's approaches". Folding@home. typepad.com. Retrieved February 25, 2012.
- ^
Thomas J. Lane, Gregory R. Bowman, Kyle A Beauchamp, Vincent Alvin Voelz, and Vijay S. Pande (2011). "Markov State Model Reveals Folding and Functional Dynamics in Ultra-Long MD Trajectories". Journal of the American Chemical Society. 133 (45): 18413–9. doi:10.1021/ja207470h. PMC 3227799. PMID 21988563.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - David E. Shaw; et al. (2009). "Millisecond-scale molecular dynamics simulations on Anton". Proceedings of the Conference on High Performance Computing Networking, Storage and Analysis (39): 1–11. doi:10.1145/1654059.1654099. ISBN 978-1-60558-744-8.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - David E. Shaw; et al. (2010). "Atomic-Level Characterization of the Structural Dynamics of Proteins". Science. 330 (6002): 341–346. Bibcode:2010Sci...330..341S. doi:10.1126/science.1187409. PMID 20947758.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - TJ Lane (Pande lab member) (June 6, 2011). "Project 7610 & 7611 in Beta". Folding@home. phpBB Group. Retrieved February 25, 2012.(registration required)
- Pande lab. "Project 7610 Description". Folding@home. Retrieved February 26, 2012.
- Imran Haque (Pande lab member) (July 13, 2011). "Re: Are my conversion for GPU flops relativly [sic] correct?". Folding@home. phpBB Group. Retrieved September 12, 2012.
- Christopher Mims (November 8, 2010). "Why China's New Supercomputer Is Only Technically the World's Fastest". Technology Review. MIT. Retrieved February 25, 2012.
- Vijay Pande (November 9, 2008). "Re: ATI and NVIDIA stats vs. PPD numbers". Folding@home. phpBB Group. Retrieved September 12, 2012.
- Vijay Pande (November 9, 2008). "Re: ATI and NVIDIA stats vs. PPD numbers". Folding@home. phpBB Group. Retrieved February 25, 2012.
- Bruce Borden (July 12, 2011). "Re: Are my conversion for GPU flops relativly [sic] correct?". Folding@home. phpBB Group. Retrieved September 12, 2012.
External links
- Folding@home homepage
- Folding@home news blog
- Folding@home support forum
- Folding@home statistics
- List of Folding@home publications
- "Futures In Biotech" episode about Folding@home
- Video of 1.5ms folding of NTL9
- OpenMM molecular dynamics library
| PlayStation 3 | |
|---|---|
| Hardware | |
| Accessories | |
| Software | |
| Network | |
| Games | |
| Media | |
| Related | |
| |
Categories:
- Distributed computing projects
- Molecular dynamics software
- Molecular modelling software
- Molecular modelling
- Simulation software
- Protein folds
- Protein structure
- Computational biology
- Mathematical and theoretical biology
- Bioinformatics
- Computational chemistry
- Hidden Markov models
- Data mining and machine learning software
- Proprietary cross-platform software
- Cross-platform software
- Windows software
- Linux science software
- Mac OS X software
- PlayStation 3 software
- 2000 introductions
- Article Feedback 5 Additional Articles