| Revision as of 06:11, 22 June 2014 editBgwhite (talk | contribs)Extended confirmed users547,151 edits WP:CHECKWIKI error fix #69. ISBN problem. Do general fixes and cleanup if needed. -, typo(s) fixed: commerical → commercial, occurence → occurrence, a Ar → an Ar using AWB (10242)← Previous edit | Revision as of 20:22, 22 June 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,701 edits rm image of R4Sn2O2, this is extremely rare, but mention in text even the trimers are unusualNext edit → | ||
| Line 15: | Line 15: | ||
| Organotin oxides and hydroxides are common products from the hydrolysis of organotin halides. Unlike the corresponding derivatives of silicon and germanium, tin oxides and hydroxides often adopt structures with penta- and hexacoordinated tin centres, especially for the diorgano- and monoorgano derivatives. Structurally simplest are the triorganotin oxides/hydroxides. | Organotin oxides and hydroxides are common products from the hydrolysis of organotin halides. Unlike the corresponding derivatives of silicon and germanium, tin oxides and hydroxides often adopt structures with penta- and hexacoordinated tin centres, especially for the diorgano- and monoorgano derivatives. Structurally simplest are the triorganotin oxides/hydroxides. | ||
| Triorganotin hydroxides are well known, e.g. the commercial ] Plictran, (])<sub>3</sub>SnOH. |
Triorganotin hydroxides are well known, e.g. the commercial ] Cyhexatin (also called Plictran), (])<sub>3</sub>SnOH. Such triorganotin hydroxides exist in equilibrium with the distannoxanes: | ||
| : 2 R<sub>3</sub>SnOH <math>\overrightarrow{\leftarrow}</math> R<sub>3</sub>SnOSnR<sub>3</sub> + H<sub>2</sub>O | : 2 R<sub>3</sub>SnOH <math>\overrightarrow{\leftarrow}</math> R<sub>3</sub>SnOSnR<sub>3</sub> + H<sub>2</sub>O | ||
| With only two organic substituents on each Sn centre, the diorganotin oxides and hydroxides form families of complex structures.<ref name=Chand>Vadapalli Chandrasekhar, Selvarajan Nagendran, Viswanathan Baskar "Organotin assemblies containing Sn/O bonds" Coordination Chemistry Reviews 2002, vol. 235, 1-52. {{DOI|10.1016/S0010-8545(02)00178-9}}</ref> The simple ]s (R<sub>2</sub>Sn(OH)<sub>2</sub>) and monomeric stannanones (R<sub>2</sub>Sn=O) are unknown. Diorganotin oxides (R<sub>2</sub>SnO) are polymers except with bulky R groups, which are cyclic trimers or dimers, i.e., Sn<sub>3</sub>O<sub>3</sub> and Sn<sub>2</sub>O<sub>2</sub>. The distannoxanes exist as dimers of dimers with the formula <sub>2</sub>O<sub>2</sub> wherein the X groups (e.g., chloride, hydroxide, carboxylate) can be terminal or bridging (see Table). The hydrolysis of the monoorganotin trihalides has the potential to generate stannanoic acids, RSnO2H. As for the diorganotin oxides/hydroxides, the monoorganotin species form structurally complex because of the occurrence of dehydration/hydration, aggregation. Illustrative is the hydrolysis of butyltin trichloride to give <sup>2+</sup>. | With only two organic substituents on each Sn centre, the diorganotin oxides and hydroxides form families of complex structures.<ref name=Chand>Vadapalli Chandrasekhar, Selvarajan Nagendran, Viswanathan Baskar "Organotin assemblies containing Sn/O bonds" Coordination Chemistry Reviews 2002, vol. 235, 1-52. {{DOI|10.1016/S0010-8545(02)00178-9}}</ref> The simple ]s (R<sub>2</sub>Sn(OH)<sub>2</sub>) and monomeric stannanones (R<sub>2</sub>Sn=O) are unknown. Diorganotin oxides are called (R<sub>2</sub>SnO) are polymers except with bulky R groups, which are cyclic trimers or, in the case of R = CH(SiMe<sub>3</sub>)<sub>2</sub> dimers, i.e., Sn<sub>3</sub>O<sub>3</sub> and Sn<sub>2</sub>O<sub>2</sub>. The distannoxanes exist as dimers of dimers with the formula <sub>2</sub>O<sub>2</sub> wherein the X groups (e.g., chloride, hydroxide, carboxylate) can be terminal or bridging (see Table). The hydrolysis of the monoorganotin trihalides has the potential to generate stannanoic acids, RSnO2H. As for the diorganotin oxides/hydroxides, the monoorganotin species form structurally complex because of the occurrence of dehydration/hydration, aggregation. Illustrative is the hydrolysis of butyltin trichloride to give <sup>2+</sup>. | ||
| {|align="center" class="wikitable" | {|align="center" class="wikitable" | ||
| |<center>] </center> || <center>]</center>||<center>]</center> | |||
| |- | |- | ||
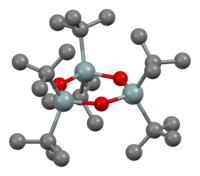
| |<center><small>Idealized structure of trimeric diorganotin oxide.</small></center> || <center><small>Ball-and-stick model for (t-Bu<sub>2</sub>SnO)<sub>3</sub>.</small></center> || <center><small>Structure of diorganotin oxide, highlighting the extensive intermolecular bonding.</small></center> | |||
| |} | |} | ||
| ====Hypercoordinated stannanes==== | ====Hypercoordinated stannanes==== | ||
| Unlike |
Unlike carbon(IV) analogues but somewhat like silicon compounds, tin(IV) can also be ] to five and even six atoms instead of the regular four. These hypercoordinated compounds usually have ] substituents. Numerous examples of hypervalency are provided by the organotin oxides and associated carboxylates and related pseudohalide derivatives.<ref name=Chand/> The organotin halides for adducts, e.g. Me<sub>2</sub>SnCl<sub>2</sub>(]. | ||
| The all-organic penta- and hexaorganostannates have even been characterized,<ref>{{cite journal | title = Lithium-Metalloid Exchange Reactions. Observation of Lithium Pentaalkyl/aryl Tin Ate Complexes | journal = ] | first2 = Nancy H. | year = 1986 | last2 = Phillips | volume = 108 | pages = 2102 | doi = 10.1021/ja00268a067 | author = Reich, Hans J.}}</ref> while in the subsequent year a six-coordinated tetraorganotin compound was reported.<ref>{{cite journal | title = Synthesis, Spectroscopic Study, and X-ray Crystal Structure of Bisdiphenyltin(IV): The First Example of a Six-Coordinate Tetraorganotin Compound | author = V. G. Kumar Das, Lo Kong Mun, Chen Wei, and Thomas C. W. Mak | journal = ] | year = 1987 | volume = 6 | pages = 10 | doi = 10.1021/om00144a003}}</ref> A crystal structure of room-temperature stable (in ]) all-carbon pentaorganostannane was reported as the ] salt with this structure:<ref>{{cite journal | title = Synthesis and Structure of Pentaorganostannate Having Five Carbon Substituents | author = Masaichi Saito, Sanae Imaizumi, Tomoyuki Tajima, Kazuya Ishimura, and Shigeru Nagase | journal = ] | year = 2007 | volume = 129 | pages = 10974–10975 | doi = 10.1021/ja072478}}</ref> | The all-organic penta- and hexaorganostannates have even been characterized,<ref>{{cite journal | title = Lithium-Metalloid Exchange Reactions. Observation of Lithium Pentaalkyl/aryl Tin Ate Complexes | journal = ] | first2 = Nancy H. | year = 1986 | last2 = Phillips | volume = 108 | pages = 2102 | doi = 10.1021/ja00268a067 | author = Reich, Hans J.}}</ref> while in the subsequent year a six-coordinated tetraorganotin compound was reported.<ref>{{cite journal | title = Synthesis, Spectroscopic Study, and X-ray Crystal Structure of Bisdiphenyltin(IV): The First Example of a Six-Coordinate Tetraorganotin Compound | author = V. G. Kumar Das, Lo Kong Mun, Chen Wei, and Thomas C. W. Mak | journal = ] | year = 1987 | volume = 6 | pages = 10 | doi = 10.1021/om00144a003}}</ref> A crystal structure of room-temperature stable (in ]) all-carbon pentaorganostannane was reported as the ] salt with this structure:<ref>{{cite journal | title = Synthesis and Structure of Pentaorganostannate Having Five Carbon Substituents | author = Masaichi Saito, Sanae Imaizumi, Tomoyuki Tajima, Kazuya Ishimura, and Shigeru Nagase | journal = ] | year = 2007 | volume = 129 | pages = 10974–10975 | doi = 10.1021/ja072478}}</ref> | ||
| Line 82: | Line 82: | ||
| == Use and toxicity == | == Use and toxicity == | ||
| * Tetraorganotins are very stable molecules with low toxicity and low biological activity. They are unusable as biocides, but they can be metabolized to toxic triorganotin compounds. They are used as starting materials for catalysts. | * Tetraorganotins are very stable molecules with low toxicity and low biological activity. They are unusable as biocides, but they can be metabolized to toxic triorganotin compounds. They are used as starting materials for catalysts. | ||
| * Triorganotins |
* Triorganotins can be very toxic. Tri-''n''-alkyltins are ] and therefore cannot be used in agriculture. Depending on the organic groups, they can be powerful ]s and ]s. ]s are used as industrial biocides, e.g. as antifungal agents in textiles and paper, wood pulp and paper mill systems, breweries, and industrial cooling systems. Tributyltins are also used in marine anti-fouling paint. Triphenyltins are used as active components of antifungal paints and agricultural fungicides. Other triorganotins are used as ]s and ]s. | ||
| * Diorganotins have no antifungal activity, low toxicity, and low antibacterial activity, except for ]s. They are used in polymer manufacturing, as ] heat stabilizers, catalysts, in the manufacturing of ] and ] curing. ] is however immunotoxic, and a recent paper suggests a link to auto-immune related diseases.<ref>{{cite journal | author = C Gumy et al. | journal = PLoS ONE | year = 2008 | pages = e3545 | volume = 3 | doi = 10.1371/journal.pone.0003545 | title = Dibutyltin Disrupts Glucocorticoid Receptor Function and Impairs Glucocorticoid-Induced Suppression of Cytokine Production|bibcode = 2008PLoSO...3.3545G }}</ref> | * Diorganotins have no antifungal activity, low toxicity, and low antibacterial activity, except for ]s. They are used in polymer manufacturing, as ] heat stabilizers, catalysts, in the manufacturing of ] and ] curing. ] is however immunotoxic, and a recent paper suggests a link to auto-immune related diseases.<ref>{{cite journal | author = C Gumy et al. | journal = PLoS ONE | year = 2008 | pages = e3545 | volume = 3 | doi = 10.1371/journal.pone.0003545 | title = Dibutyltin Disrupts Glucocorticoid Receptor Function and Impairs Glucocorticoid-Induced Suppression of Cytokine Production|bibcode = 2008PLoSO...3.3545G }}</ref> | ||
| * Monoorganotins have no biocidal activity and their toxicity to mammals is very low. Methyltin, butyltin, octyltin and monoestertins are used as PVC heat stabilizers. | * Monoorganotins have no biocidal activity and their toxicity to mammals is very low. Methyltin, butyltin, octyltin and monoestertins are used as PVC heat stabilizers. | ||
| Line 101: | Line 101: | ||
| Image:|], a very stable white crystalline solid, for control of ]s | Image:|], a very stable white crystalline solid, for control of ]s | ||
| Image:Azocyclotin.png|], a colorless crystalline solid, used as a long-acting ] for control of ]s on plants | Image:Azocyclotin.png|], a colorless crystalline solid, used as a long-acting ] for control of ]s on plants | ||
| Image:Cyhexatin.svg| |
Image:Cyhexatin.svg|Cyhexatin, a white crystalline solid, used as an ] and ] | ||
| Image:Hexamethylditin.svg|] used as an intermediate in chemical synthesis | Image:Hexamethylditin.svg|] used as an intermediate in chemical synthesis | ||
| Image:Tetraethyltin.svg|], ] 63–65° /12 mm is a catalyst<ref>], Coll. Vol. 4, p.881 (1963); Vol. 36, p.86 (1956). </ref> | Image:Tetraethyltin.svg|], ] 63–65° /12 mm is a catalyst<ref>], Coll. Vol. 4, p.881 (1963); Vol. 36, p.86 (1956). </ref> | ||
Revision as of 20:22, 22 June 2014

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.
Structure of organotin compounds
Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful.
Organic derivatives of tin(IV)
The entire series R4−nSnCln are known for many R groups and values of n up to 4. The tetraorgano derivatives are invariably tetrahedral.
Organotin halides and hydrides
The mixed organic-chloro compounds are also tetrahedral, although they form adducts with good Lewis bases such as pyridine. The fluoride tend to associate such that dimethyltin difluoride forms sheet-like polymers. The mixed organic tin hydrides, e.g. dialkyltin dihydride, are also generally monomeric. The parent member of this series, stannane (SnH4), is an unstable colourless gas.
Organotin oxides and hydroxides
Organotin oxides and hydroxides are common products from the hydrolysis of organotin halides. Unlike the corresponding derivatives of silicon and germanium, tin oxides and hydroxides often adopt structures with penta- and hexacoordinated tin centres, especially for the diorgano- and monoorgano derivatives. Structurally simplest are the triorganotin oxides/hydroxides.
Triorganotin hydroxides are well known, e.g. the commercial acaricide Cyhexatin (also called Plictran), (C6H11)3SnOH. Such triorganotin hydroxides exist in equilibrium with the distannoxanes:
- 2 R3SnOH R3SnOSnR3 + H2O
With only two organic substituents on each Sn centre, the diorganotin oxides and hydroxides form families of complex structures. The simple geminal diols (R2Sn(OH)2) and monomeric stannanones (R2Sn=O) are unknown. Diorganotin oxides are called (R2SnO) are polymers except with bulky R groups, which are cyclic trimers or, in the case of R = CH(SiMe3)2 dimers, i.e., Sn3O3 and Sn2O2. The distannoxanes exist as dimers of dimers with the formula 2O2 wherein the X groups (e.g., chloride, hydroxide, carboxylate) can be terminal or bridging (see Table). The hydrolysis of the monoorganotin trihalides has the potential to generate stannanoic acids, RSnO2H. As for the diorganotin oxides/hydroxides, the monoorganotin species form structurally complex because of the occurrence of dehydration/hydration, aggregation. Illustrative is the hydrolysis of butyltin trichloride to give .
 |
 |
 |
Hypercoordinated stannanes
Unlike carbon(IV) analogues but somewhat like silicon compounds, tin(IV) can also be coordinated to five and even six atoms instead of the regular four. These hypercoordinated compounds usually have electronegative substituents. Numerous examples of hypervalency are provided by the organotin oxides and associated carboxylates and related pseudohalide derivatives. The organotin halides for adducts, e.g. Me2SnCl2(bipyridine.
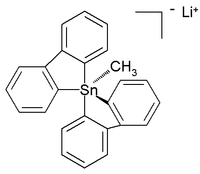
The all-organic penta- and hexaorganostannates have even been characterized, while in the subsequent year a six-coordinated tetraorganotin compound was reported. A crystal structure of room-temperature stable (in argon) all-carbon pentaorganostannane was reported as the lithium salt with this structure:
In this distorted trigonal bipyramidal structure the carbon to tin bond lengths (2.26 Å apical, 2.17 Å equatorial) are larger than regular C-Sn bonds (2.14 Å) reflecting its hypervalent nature.
Tin radicals (organic derivatives of tin(III))
Tin radicals, with the formula R3Sn, are called stannyl radicals. They are invoked as intermediates in certain atom-transfer reactions. For example, tributyltin hydride (tri-n-butylstannane) serves as a useful source of "hydrogen atoms" because of the stability of the tributytin radical.
Organic derivatives of tin(II)
Organotin(II) compounds are somewhat rare. Compounds with the empirical formula SnR2 are somewhat fragile and exist as rings or polymers when R is not bulky. The polymers, called polystannanes, have the formula (SnR2)n.
In principle divalent tin compounds might be expected to form analogues of alkenes with a formal double bond. Indeed compounds with the formula Sn2R4, called distannenes, are known for certain organic substituents. The Sn centres tend to be highly pyramidal. Monomeric compounds with the formula SnR2, analogues of carbenes are also known in a few cases. One example is Sn(SiR3)2, where R is the very bulky CH(SiMe3)2 (Me = methyl). Such species reversibly dimerize to the distannylene upon crystallization:
- 2 R2Sn (R2Sn)2
Stannenes, compounds with tin–carbon double bonds, are exemplified by derivatives of stannabenzene. Stannoles, structural analogs of cyclopentadiene, exhibit little C-Sn double bond character.
Organic derivatives of tin(I)
Compounds of Sn(I) are rare and only observed with very bulky ligands. One prominent family of cages is accessed by pyrolysis of the 2,6-diethylphenyl-substituted tristannylene 3, which affords the cubane and a prismane. These cages contain Sn(I) and have the formula n where n = 8, 10. A stannyne contains a carbon to tin triple bond and a distannyne a triple bond between two tin atoms (RSnSnR). Distannynes only exist for extremely bulky substituents. Unlike alkynes, the C-Sn-Sn-C core of these distannynes are nonlinear, although they are planar. The Sn-Sn distance is 3.066(1) Å, and the Sn-Sn-C angles are 99.25(14)°. Such compounds are prepared by reduction of bulky aryltin(II) halides.

Preparation of organotin compounds
Organotin compounds can be synthesised by numerous methods. Classic is the reaction of a Grignard reagent with tin halides for example tin tetrachloride. An example is provided by the synthesis of tetraethyltin:
- 4 EtMgBr + SnCl4 → Et4Sn + 4 MgClBr
The symmetrical tetraorganotin compounds can then be converted to various mixed chlorides by redistribution reactions (also known as the "Kocheshkov comproportionation"):
- 3 R4Sn + SnCl4 → 4 R3SnCl
- R4Sn + SnCl4 → 2 R2SnCl2
- R4Sn + 3 SnCl4 → 4 RSnCl3
A related method involves redistribution of tin halides with organoaluminium compounds.
The mixed organo-halo tin compounds can be converted to the mixed organic derivatives, as illustrated by the synthesis of dibutyldivinyltin:
- Bu2SnCl2 + 2 C2H3MgBr → Bu2Sn(C2H3)2 + 2 MgBrCl
The organotin hydrides are generated by reduction of the mixed alkyl chlorides. For example, treatment of dibutyltin dichloride with lithium aluminium hydride gives the dibutyltin dihydride, a colourless distillable oil:
- Bu2SnCl2 + 1/2 LiAlH4 → Bu2SnH2 + 1/2 LiAlCl4"
The Wurtz-like coupling of alkyl sodium compounds with tin halides yields tetraorganotin compounds.
Reactions of organotin compounds
Important reactions involving organotin compounds are the Stille reaction (coupling reaction with sp2-hybridized organic halides catalyzed by palladium):
and organostannane additions (nucleophilic addition of an allyl-, allenyl-, or propargylstannanes to an aldehydes and imines). Organotin compounds are also used extensively in radical chemistry (e.g. radical cyclizations, Barton–McCombie deoxygenation, Barton decarboxylation, etc.).
Use and toxicity
- Tetraorganotins are very stable molecules with low toxicity and low biological activity. They are unusable as biocides, but they can be metabolized to toxic triorganotin compounds. They are used as starting materials for catalysts.
- Triorganotins can be very toxic. Tri-n-alkyltins are phytotoxic and therefore cannot be used in agriculture. Depending on the organic groups, they can be powerful bactericides and fungicides. Tributyltins are used as industrial biocides, e.g. as antifungal agents in textiles and paper, wood pulp and paper mill systems, breweries, and industrial cooling systems. Tributyltins are also used in marine anti-fouling paint. Triphenyltins are used as active components of antifungal paints and agricultural fungicides. Other triorganotins are used as miticides and acaricides.
- Diorganotins have no antifungal activity, low toxicity, and low antibacterial activity, except for diphenyltins. They are used in polymer manufacturing, as PVC heat stabilizers, catalysts, in the manufacturing of polyurethane and silicone curing. DBT is however immunotoxic, and a recent paper suggests a link to auto-immune related diseases.
- Monoorganotins have no biocidal activity and their toxicity to mammals is very low. Methyltin, butyltin, octyltin and monoestertins are used as PVC heat stabilizers.
- Many different organotin complexes are being studied in anticancer therapy, observing that their cytotoxicity and selectivity towards cancer cell is higher than that of cisplatin.
Applications
An organotin compound is commercially applied as a hydrochloric acid scavenger (or heat stabilizer) in polyvinyl chloride and as a biocide. Tributyltin oxide has been extensively used as a wood preservative. Tributyltin compounds are used as marine anti-biofouling agents. Concerns over toxicity of these compounds (some reports describe biological effects to marine life at a concentration of 1 nanogram per liter) have led to a worldwide ban by the International Maritime Organization. n-Butyltin trichloride is used in the production of tin dioxide layers on glass bottles by chemical vapor deposition.
Compounds
Organotin compounds are used commercially in a wide range of applications such as biocides, insecticides, chemical intermediates and as catalysts.
- Organotin compounds
-
 Tetrabutyltin starting material for the di- and tributyl compounds
Tetrabutyltin starting material for the di- and tributyl compounds
-
 Tributyltin oxide, a colorless to pale yellow liquid used in wood preservation
Tributyltin oxide, a colorless to pale yellow liquid used in wood preservation
-
 Triphenyltin acetate, an off-white crystalline solid, used as an insecticide and a fungicide
Triphenyltin acetate, an off-white crystalline solid, used as an insecticide and a fungicide
-
 Triphenyltin chloride, a white crystalline solid, used as a biocide and an intermediate in chemical synthesis
Triphenyltin chloride, a white crystalline solid, used as a biocide and an intermediate in chemical synthesis
-
 Trimethyltin chloride also a biocide
Trimethyltin chloride also a biocide
-
 Triphenyltin hydroxide, an off-white powder, used as a fungicide and to sterilize insects
Triphenyltin hydroxide, an off-white powder, used as a fungicide and to sterilize insects
-
 Azocyclotin, a colorless crystalline solid, used as a long-acting acaricide for control of spider mites on plants
Azocyclotin, a colorless crystalline solid, used as a long-acting acaricide for control of spider mites on plants
-
 Cyhexatin, a white crystalline solid, used as an acaricide and miticide
Cyhexatin, a white crystalline solid, used as an acaricide and miticide
-
 Hexamethylditin used as an intermediate in chemical synthesis
Hexamethylditin used as an intermediate in chemical synthesis
-
 Tetraethyltin, boiling point 63–65° /12 mm is a catalyst
Tetraethyltin, boiling point 63–65° /12 mm is a catalyst
See also
| Compounds of carbon with other elements in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Legend |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ^ Davies, Alwyn George. (2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. ISBN 978-3-527-31023-4
- ^ Vadapalli Chandrasekhar, Selvarajan Nagendran, Viswanathan Baskar "Organotin assemblies containing Sn/O bonds" Coordination Chemistry Reviews 2002, vol. 235, 1-52. doi:10.1016/S0010-8545(02)00178-9
- Reich, Hans J.; Phillips, Nancy H. (1986). "Lithium-Metalloid Exchange Reactions. Observation of Lithium Pentaalkyl/aryl Tin Ate Complexes". J. Am. Chem. Soc. 108: 2102. doi:10.1021/ja00268a067.
- V. G. Kumar Das, Lo Kong Mun, Chen Wei, and Thomas C. W. Mak (1987). "Synthesis, Spectroscopic Study, and X-ray Crystal Structure of Bisdiphenyltin(IV): The First Example of a Six-Coordinate Tetraorganotin Compound". Organometallics. 6: 10. doi:10.1021/om00144a003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Masaichi Saito, Sanae Imaizumi, Tomoyuki Tajima, Kazuya Ishimura, and Shigeru Nagase (2007). "Synthesis and Structure of Pentaorganostannate Having Five Carbon Substituents". J. Am. Chem. Soc. 129: 10974–10975. doi:10.1021/ja072478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - T. V. RajanBabu, P. C. B. Page B. R. Buckley "Tri-n-butylstannane" in e-EROS Encyclopedia of Reagents for Organic Synthesis, 2004. doi:10.1002/047084289X.rt181.pub2
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- Lawrence R. Sita "Heavy-Metal Organic Chemistry: Building with Tin" Acc. Chem. Res., 1994, volume 27, pp 191–197. doi:10.1021/ar00043a002
- Philip P. Power "Bonding and Reactivity of Heavier Group 14 Element Alkyne Analogues" Organometallics 2007, volume 26, pp 4362–4372. doi:10.1021/om700365p
- Sander H.L. Thoonen, Berth-Jan Deelman, Gerard van Koten (2004). "Synthetic aspects of tetraorganotins and organotin(IV) halides" (PDF). Journal of Organometallic Chemistry (689): 2145–2157.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - G. J. M. Van Der Kerk, J. G. A. Luijten "Tetraethyltin" Org. Synth. 1956, volume 36, page 86ff. doi:10.15227/orgsyn.036.0086
- Dietmar Seyferth "Di-n-butyldivinyltin" Org. Synth. 1959, volume 39, page 10. doi:10.15227/orgsyn.039.0010
- "Organometallic Syntheses: Nontransition-Metal Compounds" John Eisch, Ed. Academic Press: New York, 1981. ISBN 0122349504.
- C Gumy; et al. (2008). "Dibutyltin Disrupts Glucocorticoid Receptor Function and Impairs Glucocorticoid-Induced Suppression of Cytokine Production". PLoS ONE. 3: e3545. Bibcode:2008PLoSO...3.3545G. doi:10.1371/journal.pone.0003545.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: unflagged free DOI (link) - S. Gómez-Ruiz; et al. (2008). "Study of the cytotoxic activity of di and triphenyltin(IV) carboxylate complexes". Journal of Inorganic Biochemistry. 102 (12): 2087. doi:10.1016/j.jinorgbio.2008.07.009. PMID 18760840.
{{cite journal}}: Explicit use of et al. in:|author=(help) - Gajda, M. (2010). "Organotins, formation, use, speciation and toxicology". Metal ions in life sciences. 7, Organometallics in environment and toxicology. Cambridge: RSC publishing. ISBN 9781847551771.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Organic Syntheses, Coll. Vol. 4, p.881 (1963); Vol. 36, p.86 (1956). Link
External links
- National Pollutant Inventory Fact Sheet for organotins
- Industry information site
- Organotin chemistry in synthesis
- EU bans certain organotin compounds in consumer products
| Health issues of plastics and polyhalogenated compounds (PHCs) | |
|---|---|
| Plasticizers: Phthalates | |
| Miscellaneous plasticizers | |
| Monomers |
|
| Miscellaneous additives incl. PHCs | |
| Health issues | |
| Pollution | |
| Regulations | |
 R3SnOSnR3 + H2O
R3SnOSnR3 + H2O