| Revision as of 15:27, 26 July 2005 editTexasAndroid (talk | contribs)109,350 edits disambiguation link repair (You can help!)← Previous edit | Latest revision as of 16:22, 22 October 2024 edit undoCitation bot (talk | contribs)Bots5,429,656 edits Alter: template type, date. Add: doi-access, pages, issue, volume, date, journal, authors 1-2. Removed URL that duplicated identifier. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Metamd | #UCB_webform | ||
| (244 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| '''Grubbs catalysts''' are a series of ]es used as ]s for ]. They are named after ], the chemist who supervised their synthesis. Several generations of the ] have also been developed.<ref>{{cite book | last = Grubbs | first = Robert H. | title = Handbook of Metathesis | publisher = Wiley-VCH | location = Weinheim | year = 2003 | isbn = 978-3-527-30616-9 | edition = 1st}}</ref><ref>{{cite encyclopedia|last1=Grubbs|first1=R. H.|pages=153–177|last2=Trnka|first2=T. M.|title=Ruthenium-Catalyzed Olefin Metathesis |doi=10.1002/3527603832.ch6|encyclopedia=Ruthenium in Organic Synthesis|editor-first=S.|editor-last=Murahashi|location=Weinheim|publisher=Wiley-VCH|date=2004|isbn=978-3-527-60383-1}}</ref> Grubbs catalysts tolerate many ] in the ] substrates, are air-tolerant, and are compatible with a wide range of solvents.<ref name=vougioukalakis>{{cite journal |last1=Vougioukalakis |first1=G. C. |last2=Grubbs|first2= R. H. |year=2010 |title=Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts |journal=] |pmid=20000700 |volume=110 |issue=3 |pages=1746–1787 |doi=10.1021/cr9002424}} | |||
| {| align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse" | |||
| </ref><ref>{{cite journal|title=The Development of L<sub>2</sub>X<sub>2</sub>Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story| last1 = Trnka | first1 = T. M. | last2 = Grubbs | first2 = R. H. |journal= ]| year=2001| volume=34| issue=1| pages= 18–29 | doi=10.1021/ar000114f | pmid=11170353}}</ref> For these reasons, Grubbs catalysts have become popular in ].<ref>{{cite book | title = Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysts | last1 = Cossy | first1 = Janine | last2 = Arseniyadis | first2 = Stellios | last3 = Meyer | first3 = Christophe | publisher = Wiley-VCH | location = Weinheim | year = 2010 | isbn = 978-3-527-32440-8 | edition = 1st}}</ref> Grubbs, together with ] and ], won the ] in recognition of their contributions to the development of olefin metathesis. | |||
| ! bgcolor="#ffddaa" colspan="2" | Grubbs' Catalyst 1<sup>st</sup> Generation | |||
| |- | |||
| | align="center" colspan="2" | ] | |||
| |- | |||
| ! bgcolor="#ffddaa" colspan="2" | General | |||
| |- | |||
| | ] | |||
| | C<sub>43</sub>H<sub>72</sub>Cl<sub>2</sub>P<sub>2</sub>Ru | |||
| |- | |||
| | ] | |||
| | 822.96 g/mol | |||
| |- | |||
| | Appearance | |||
| | Purple Solid | |||
| |- | |||
| | ] | |||
| | | |||
| |- | |||
| | ] | |||
| | 153 °C (426 K) | |||
| |- | |||
| | | |||
| |- | |||
| ! bgcolor="#ffddaa" colspan="2" | Grubbs' Catalyst 2<sup>nd</sup> Generation | |||
| |- | |||
| | align="center" colspan="2" | ] | |||
| |- | |||
| ! bgcolor="#ffddaa" colspan="2" | General | |||
| |- | |||
| | ] | |||
| | C<sub>46</sub>H<sub>65</sub>Cl<sub>2</sub>N<sub>2</sub>PRu | |||
| |- | |||
| | ] | |||
| | 848.97 g/mol | |||
| |- | |||
| | Appearance | |||
| | Pinkish-Brown Solid | |||
| |- | |||
| | ] | |||
| | | |||
| |- | |||
| | ] | |||
| | 143.5-148.5 °C (416.5-421.5 K) | |||
| |- | |||
| |} | |||
| ==First-generation Grubbs catalyst== | |||
| {{Chembox | |||
| |Verifiedfields = changed | |||
| |Watchedfields = changed | |||
| |verifiedrevid = 447372032 | |||
| |Name = First-generation Grubbs catalyst | |||
| |ImageFile = Grubbs Catalyst 1st Generation.svg | |||
| |ImageFile1 = Grubbs-1G-from-xtal-2010-3D-balls.png | |||
| |IUPACName = Benzylidene-bis(tricyclohexylphosphino)-dichlororuthenium | |||
| |OtherNames = | |||
| |Section1={{Chembox Identifiers | |||
| |CASNo_Ref = {{cascite|correct|??}} | |||
| |CASNo = 172222-30-9 | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| |UNII = J7P585D3ZC | |||
| |PubChem = 86306055 | |||
| |ChemSpiderID = 25071160 | |||
| |InChI=1S/C43H72P2.2ClH.Ru/c1-8-22-36(23-9-1)43(44(37-24-10-2-11-25-37,38-26-12-3-13-27-38)39-28-14-4-15-29-39)45(40-30-16-5-17-31-40,41-32-18-6-19-33-41)42-34-20-7-21-35-42;;;/h1,8-9,22-23,37-43H,2-7,10-21,24-35H2;2*1H;/q+2;;;+2/p-2 | |||
| |InChIKey = NDDFAYQFCZRYDT-UHFFFAOYSA-L | |||
| |SMILES = Cl(Cl)((C1CCCCC1)(C1CCCCC1)C1CCCCC1)((C1CCCCC1)(C1CCCCC1)C1CCCCC1)=Cc1ccccc1 | |||
| }} | |||
| |Section2={{Chembox Properties | |||
| |C=43 | H=72 | Cl=2 | P=2 | Ru=1 | |||
| |Appearance = Purple solid | |||
| |MeltingPtC = 153 | |||
| |MeltingPt_notes = (decomposition) | |||
| |Solubility = | |||
| }} | |||
| }} | |||
| In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use.<ref name=KO/> The first well-defined ruthenium catalyst was reported in 1992.<ref>{{cite journal | last1 = Nguyen | first1 = S. T. | last2 = Johnson | first2 = L. K. | last3 = Grubbs | first3 = R. H. | last4 = Ziller | first4 = J. W. | title = Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media | journal = ] | volume = 114 | issue=10 | pages = 3974–3975 | year = 1992 | doi = 10.1021/ja00036a053| url = https://authors.library.caltech.edu/88217/2/ja00036a053_si_001.pdf}}</ref> It was prepared from RuCl<sub>2</sub>(PPh<sub>3</sub>)<sub>4</sub> and diphenylcyclopropene. | |||
| '''Grubbs' Catalyst''' is named after the chemist by whom it was first synthesized, Robert H. Grubbs. There are two generations of the catalyst, as shown on the right. In contrast to other ] catalysts, Grubbs' Catalysts tolerate other ] in the ] and are compatible with a wide range of solvents<sup>4</sup>. For these reasons, Grubbs' Catalysts are extraordinarily versatile. | |||
| ] | |||
| The 1<sup>st</sup> Generation ] is often used in ] to achieve ] (see below), ] (ROMP), and ]<sup>2</sup>. It is easily synthesized from RuCl<sub>2</sub>(PPh<sub>3</sub>)<sub>3</sub>, ], and tricyclohexylphosphine in a ] <sup>1</sup>. Grubbs' Catalyst is a relatively stable compound in air, which makes handling very easy. The ] name of the 1<sup>st</sup> Generation Catalyst is benzylidene-bis(tricyclohexylphosphine)dichlororuthenium. | |||
| This initial ruthenium catalyst was followed in 1995 by what is now known as the first-generation Grubbs catalyst. It is synthesized from ], ], and ] in a ].<ref>{{cite journal | journal = ] | title = A Series of Well-Defined Metathesis Catalysts – Synthesis of and Its Reactions | volume = 34 | pages = 2039–2041 | issue = 18 | last1 = Schwab | first1 = P. | last2 = France | first2 = M. B. | last3 = Ziller | first3 = J. W. | last4 = Grubbs | first4 = R. H. | doi = 10.1002/anie.199520391| year = 1995}}</ref><ref name=schwab2>{{cite journal| title=Synthesis and Applications of RuCl<sub>2</sub>(=CHR′)(PR<sub>3</sub>)<sub>2</sub>: The Influence of the Alkylidene Moiety on Metathesis Activity | last1=Schwab|first1= P.|last2= Grubbs|first2= R. H.|last3= Ziller|first3= J. W. |journal=J. Am. Chem. Soc.| year=1996 |volume=118 |issue=1| pages=100–110| doi=10.1021/ja952676d}}</ref> | |||
| ], shown below, is a reaction between two molecules containing double bonds. The groups bonded to the carbon atoms of the double bond are exchanged between molecules, to produce two new molecules containing double bonds with swapped groups. Whether a ] or ] ] is formed in this type of reaction is determined by the orientation the molecules assume when they coordinate to the catalyst, as well as the ] of the ]s on the double bond of the newly forming molecule. Other catalysts are effective for this reaction, notably those developed by Schrock (]). | |||
| ] | |||
| The first-generation Grubbs catalyst was the first well-defined Ru-based catalyst. It is also important as a precursor to all other Grubbs-type catalysts. | |||
| The 2<sup>nd</sup> Generation Catalyst has the same uses in organic synthesis as the 1<sup>st</sup> Generation Catalyst, but has a higher activity. This catalyst is also air stable and is easily synthesized from the combination of the 1<sup>st</sup> Generation Catalyst and alkoxy-protected 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene<sup>3</sup>. The ] name of the 2<sup>nd</sup> Generation Catalyst is benzylidenedichloro(tricyclohexylphosphine)ruthenium. Both generations of the catalyst are commercially available through . | |||
| ==Second-generation Grubbs catalyst== | |||
| {{Chembox | |||
| |Name = Second-generation Grubbs catalyst | |||
| |ImageFile = Grubbs catalyst Gen2.svg | |||
| |ImageFile1 = Grubbs-2G-from-xtal-2005-3D-balls.png | |||
| |IUPACName = dichloro(phenylmethylene)(tricyclohexylphosphino)ruthenium | |||
| |Section1 = {{Chembox Identifiers | |||
| |CASNo = 246047-72-3 | |||
| |PubChem = 11147261 | |||
| |ChemSpiderID = 9322369 | |||
| |StdInChI=1S/C21H26N2.C18H33P.C7H6.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1-6H;2*1H;/q;;;;;+2/p-2 | |||
| |StdInChIKey = FCDPQMAOJARMTG-UHFFFAOYSA-L | |||
| |SMILES = Cl(Cl)(0n(-c1c(C)cc(C)cc1C)CCn0-c1c(C)cc(C)cc1C)((C1CCCCC1)(C1CCCCC1)C1CCCCC1)=Cc1ccccc1 | |||
| }} | |||
| |Section2 = {{Chembox Properties | |||
| |C=46 | H=65 | Cl=2 | N=2 | P=1 | Ru=1 | |||
| |Appearance = Pinkish brown solid | |||
| |MeltingPtC = 143.5 to 148.5 | |||
| }} | |||
| |Section3 = {{Chembox Hazards | |||
| |GHSPictograms = {{GHS02}} | |||
| |GHSSignalWord = Warning | |||
| |HPhrases = {{H-phrases|228}} | |||
| |PPhrases = {{P-phrases|210|240|241|280|378}} | |||
| }} | |||
| }} | |||
| The second-generation catalyst has the same uses in organic synthesis as the first generation catalyst, but generally with higher activity. This catalyst is stable toward ] and ], thus is easier to handle in laboratories. | |||
| An interesting application of Grubbs' Catalyst is in the aerospace industry. A spaceship's ] is a necessarily very strong material, but over time small ]s in the structure can form. A new material used in the construction of spaceship hulls contains Grubbs' Catalyst, as well as capsules of ], which can undergo ROMP. When a crack in the hull forms, the capsules are ruptured and come into contact with Grubbs' Catalyst, which ] dicyclopentadiene and seals the crack<sup>5</sup>. <br><br><br> | |||
| Shortly before the discovery of the second-generation Grubbs catalyst, a very similar catalyst based on an unsaturated ''N''-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)imidazole) was reported independently by Nolan<ref>{{cite journal|title=Olefin Metathesis-Active Ruthenium Complexes Bearing a Nucleophilic Carbene Ligand | last1 = Huang | first1 = J.-K. | last2 = Stevens | first2 = E. D. | last3 = Nolan | first3 = S. P. | last4 = Petersen | first4 = J. L. | journal= J. Am. Chem. Soc. | year=1999 | volume=121 | issue=12 | pages=2674–2678 | doi=10.1021/ja9831352}}</ref> and Grubbs<ref>{{cite journal | journal = ] | year = 1999 | volume = 40 | issue = 12 | pages = 2247–2250 | title = Increased Ring Closing Metathesis Activity of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with Imidazolin-2-ylidene Ligands | last1 = Scholl | first1 = M. | last2 = Trnka | first2 = T. M. | last3 = Morgan | first3 = J. P. | last4 = Grubbs | first4 = R. H. | doi = 10.1016/S0040-4039(99)00217-8}}</ref> in March 1999, and by Fürstner<ref>{{cite journal | journal = Tetrahedron Lett. | title = Ruthenium Carbene Complexes with Imidazolin-2-ylidene Ligands Allow the Formation of Tetrasubstituted Cycloalkenes by RCM | year = 1999 | volume = 40 | issue = 26 | pages = 4787–4790 | doi = 10.1016/S0040-4039(99)00919-3 | last1 = Ackermann | first1 = L. | last2 = Fürstner | first2 = A. | last3 = Weskamp | first3 = T. | last4 = Kohl | first4 = F. J. | last5 = Herrmann | first5 = W. A.}}</ref> in June of the same year. Shortly thereafter, in August 1999, Grubbs reported the second-generation catalyst, based on a saturated ''N''-heterocyclic carbene (]):<ref name=scholl>{{cite journal|title=Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands|last1=Scholl|first1=M.|last2=Ding|first2= S.|last3= Lee|first3= C. W.|last4= Grubbs|first4= R. H. |journal=] |year=1999| volume=1 |issue=6| pages=953–956| doi=10.1021/ol990909q|pmid=10823227}}</ref> | |||
| ] | |||
| ] | |||
| In both the saturated and unsaturated cases a phosphine ] is replaced with an ] (NHC), which is characteristic of all second-generation-type catalysts.<ref name=vougioukalakis/> | |||
| Both the first- and second-generation catalysts are commercially available, along with many derivatives of the second-generation catalyst. | |||
| == Hoveyda–Grubbs catalysts == | |||
| {{Chembox | |||
| |Name = First-generation Hoveyda–Grubbs catalyst | |||
| |ImageFile = Hoveyda-katalysator.svg | |||
| |ImageSize = 140px | |||
| |ImageFile1 = Hoveyda-Grubbs-catalyst-1st-gen 3D-balls.png | |||
| |ImageSize1 = 220px | |||
| |IUPACName = Dichloro(''o''-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium(II) | |||
| |Section1={{Chembox Identifiers | |||
| |CASNo = 203714-71-0 | |||
| |PubChem = 24880901 | |||
| |ChemSpiderID = 9116251 | |||
| |StdInChI=1S/C18H33P.C10H12O.2ClH.Ru/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-8(2)11-10-7-5-4-6-9(10)3;;;/h16-18H,1-15H2;3-8H,1-2H3;2*1H;/q;;;;+2/p-2 | |||
| |StdInChIKey = KMKCJXPECJFQPQ-UHFFFAOYSA-L | |||
| |SMILES = Cl2(Cl)((C1CCCCC1)(C1CCCCC1)C1CCCCC1)=Cc1ccccc12C(C)C | |||
| }} | |||
| |Section2={{Chembox Properties | |||
| |C=28 | H=45 | Cl=2 | P=1 | O=1 | Ru=1 | |||
| |Appearance = Brown solid | |||
| | MeltingPtC = 195 to 197 | |||
| }} | |||
| |Section3={{Chembox Hazards | |||
| |GHSPictograms = {{GHS02}} | |||
| |GHSSignalWord = Warning | |||
| |HPhrases = {{H-phrases|228}} | |||
| |PPhrases = {{P-phrases|210|240|241|280|378}} | |||
| }} | |||
| }} | |||
| {{Chembox | |||
| |Name = Second-generation Hoveyda–Grubbs catalyst | |||
| |ImageFile = Misplaced Pages-HoveydaGrubbsCatalysts.png | |||
| |ImageFile1 = Hoveyda-Grubbs-catalyst-from-xtal-2007-3D-balls.png | |||
| |IUPACName = dichloro(''o''-isopropoxyphenylmethylene)ruthenium | |||
| |Section1={{Chembox Identifiers | |||
| |CASNo = 301224-40-8 | |||
| |PubChem = 11763533 | |||
| |ChemSpiderID = 9938229 | |||
| |EC_number = 608-446-3 | |||
| |StdInChI=1S/C21H26N2.C10H12O.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-8(2)11-10-7-5-4-6-9(10)3;;;/h9-12H,7-8H2,1-6H3;3-8H,1-2H3;2*1H;/q;;;;+2/p-2 | |||
| |StdInChIKey = ZRPFJAPZDXQHSM-UHFFFAOYSA-L | |||
| |SMILES = Cl2(Cl)(0n(-c1c(C)cc(C)cc1C)CCn0-c1c(C)cc(C)cc1C)=Cc1ccccc12C(C)C | |||
| }} | |||
| |Section2={{Chembox Properties | |||
| |C=31 | H=38 | Cl=2 | N=2 | O=1 | Ru=1 | |||
| |Appearance = Green solid | |||
| |MeltingPtC = 216 to 220 | |||
| }} | |||
| }} | |||
| In the '''Hoveyda–Grubbs catalysts''', the benzylidene ligands have a chelating ''ortho''-isopropoxy group attached to the benzene rings. The ''ortho''-isopropoxybenzylidene moiety is sometimes referred to as a Hoveyda chelate. The chelating oxygen atom replaces a ] ligand, which in the case of the 2nd generation catalyst, gives a completely phosphine-free structure. The 1st generation Hoveyda–Grubbs catalyst was reported in 1999 by ]'s group,<ref>{{cite journal|last1=Kingsbury|first1=Jason S.|last2=Harrity|first2=Joseph P. A. |last3=Bonitatebus|first3=Peter J. |last4= Hoveyda|first4= Amir H. |title=A Recyclable Ru-Based Metathesis Catalyst|journal=]|date=1999|volume=121|issue=4|pages=791–799|doi=10.1021/ja983222u}}</ref> and in the following year, the second-generation Hoveyda–Grubbs catalyst was described in nearly simultaneous publications by the Blechert<ref name=":0" /> and Hoveyda<ref name="2ndGenHoveyda" /> laboratories. ]'s name is not commonly included in the eponymous catalyst name. The Hoveyda–Grubbs catalysts, while more expensive and slower to initiate than the Grubbs catalyst from which they are derived, are popular because of their improved stability.<ref name=vougioukalakis/><ref>{{Cite journal |title=Hoveyda-Grubbs type complexes with ruthenium-pnictogen/halcogen/halogen coordination bond. Synthesis, catalytic activity, applications. |url=https://rcr.colab.ws/publications/10.59761/RCR5132 |access-date=2024-10-22 |journal=Russian Chemical Reviews |date=2024 |language=en |doi=10.59761/rcr5132 |last1=Antonova |first1=A. S. |last2=Zubkov |first2=F. I. |volume=93 |issue=8 |pages=RCR5132 }}</ref> By changing the steric and electronic properties of the chelate, the initiation rate of the catalyst can be modulated,<ref>{{cite journal|last1=Engle|first1=Keary M.|last2=Lu|first2=Gang|last3=Luo|first3=Shao-Xiong|last4=Henling|first4=Lawrence M.|last5=Takase|first5=Michael K.|last6=Liu|first6=Peng|last7=Houk|first7=K. N.|last8=Grubbs|first8=Robert H.|title=Origins of Initiation Rate Differences in Ruthenium Olefin Metathesis Catalysts Containing Chelating Benzylidenes|journal=Journal of the American Chemical Society|date=2015|volume=137|issue=17|pages=5782–5792|doi=10.1021/jacs.5b01144|pmid=25897653|url=https://resolver.caltech.edu/CaltechAUTHORS:20150428-085409439 }}</ref><ref>{{cite journal|last2=Engle|first2=Keary M.|last1=Luo|first1=Shao-Xiong|last3=Deng|first3=Xiaofei|last4=Hejl|first4=Andrew|last6=Henling|first6=Lawrence M.|last5=Takase|first5=Michael K.|last7=Liu|first7=Peng|last8=Houk|first8=K. N.|last9=Grubbs|first9=Robert H.|title=An Initiation Kinetics Prediction Model Enables Rational Design of Ruthenium Olefin Metathesis Catalysts Bearing Modified Chelating Benzylidenes|journal=]|date=2018|volume=8|issue=5|pages=4600–4611|doi=10.1021/acscatal.8b00843|pmid=32528741 |pmc=7289044}}</ref> such as in the ]. Hoveyda–Grubbs catalysts are easily formed from the corresponding Grubbs catalyst by the addition of the chelating ligand and the use of a phosphine scavenger like ]:<ref name=2ndGenHoveyda>{{cite journal | journal = Journal of the American Chemical Society | title = Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts | year = 2000 | volume = 122 | issue = 34 | pages = 8168–8179 | last1 = Garber | first1 = S. B. | last2 = Kingsbury | first2 = J. S. | last3 = Gray | first3 = B. L. | last4 = Hoveyda | first4 = A. H. | doi = 10.1021/ja001179g}}</ref> | |||
| The second-generation Hoveyda–Grubbs catalysts can also be prepared from the 1st generation Hoveyda–Grubbs catalyst by the addition of the NHC:<ref name=":0">{{cite journal | journal = Tetrahedron Letters | title = Synthesis and metathesis reactions of phosphine-free dihydroimidazole carbene ruthenium complex | year = 2000 | volume = 41 | pages = 9973–9976 | issue = 51 | last1 = Gessler | first1 = S. | last2 = Randl | first2 = S. | last3 = Blechert | first3 = S. | doi = 10.1016/S0040-4039(00)01808-6}}</ref> | |||
| ] | |||
| ] | |||
| In one study published by Grubbs and Hong in 2006, a water-soluble Grubbs catalyst was prepared by attaching a ] chain to the ] group.<ref name=Grubbs-Hong2006>{{cite journal|title=Highly Active Water-Soluble Olefin Metathesis Catalyst|first1=Robert H. |last1=Grubbs|first2=Soon Hyeok|last2=Hong|journal= Journal of the American Chemical Society| year=2006| volume=128|issue=11 |pages=3508–3509| doi=10.1021/ja058451c|pmid=16536510| url=https://authors.library.caltech.edu/76728/2/ja058451csi20051213_124352.pdf}}</ref> This catalyst is used in the ] reaction in water of a diene carrying an ] group making it water-soluble as well. | |||
| <br><br><br> | |||
| ] | |||
| == Third-generation Grubbs catalyst (fast-initiating catalysts) == | |||
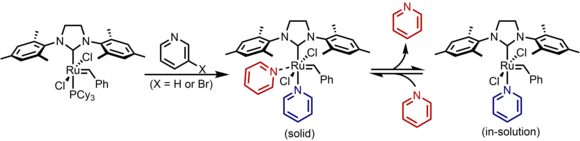
| The rate of the Grubbs catalyst can be altered by replacing the phosphine ligand with more labile ] ligands. By using ] the initiation rate is increased more than a millionfold.<ref>{{cite journal | last1 = Love | first1 = J. A. | last2 = Morgan | first2 = J. P. | last3 = Trnka | first3 = T. M. | last4 = Grubbs | first4 = R. H. | title = A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile | journal = ] | year = 2002 | volume = 41 | issue = 21 | pages = 4035–4037 | doi = 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I| pmid = 12412073}}</ref> Both pyridine and 3-bromopyridine are commonly used, with the bromo- version 4.8 times more labile resulting in even faster rates.<ref>{{Cite journal|last1=Walsh|first1=Dylan J.|last2=Lau|first2=Sii Hong|last3=Hyatt|first3=Michael G.|last4=Guironnet|first4=Damien|date=2017-09-25|title=Kinetic Study of Living Ring-Opening Metathesis Polymerization with Third-Generation Grubbs Catalysts|journal=Journal of the American Chemical Society|language=EN|volume=139|issue=39|pages=13644–13647|doi=10.1021/jacs.7b08010|pmid=28944665}}</ref> The catalyst is traditionally isolated as a two pyridine complex, however one pyridine is lost upon dissolving and ] the ] center throughout any chemical reaction. | |||
| :] | |||
| ==References== | |||
| 1. Schwab, P.; Grubbs, R. H.; Ziller, J. W. ''Journal of the American Chemical Society'', '''1996''', ''118'', 100-110. | |||
| The principal application of the fast-initiating catalysts is as initiators for ] (ROMP). Because of their usefulness in ROMP these catalysts are sometimes referred to as the 3rd generation Grubbs catalysts.<ref>{{cite journal | last1 = Leitgeb | first1 = Anita | last2 = Wappel | first2 = Julia | last3 = Slugovc | first3 = Christian | title = The ROMP toolbox upgraded | journal = Polymer | year = 2010 | volume = 51 | issue = 14 | pages = 2927–2946 | doi = 10.1016/j.polymer.2010.05.002 | doi-access = free}}</ref> The high ratio of the rate of initiation to the rate of propagation makes these catalysts useful in ], yielding polymers with low ].<ref>{{cite journal | last1 = Choi | first1 = T.-L. | last2 = Grubbs | first2 = R. H. | title = Controlled Living Ring-Opening-Metathesis Polymerization by a Fast-Initiating Ruthenium Catalyst | journal = Angewandte Chemie International Edition | year = 2003 | volume = 42 | issue = 15 | pages = 1743–1746 | doi = 10.1002/anie.200250632| pmid = 12707895}}</ref> | |||
| 2. Louie, J.; Grubbs, R. H. ''Organometallics'', '''2002''', ''21'', 2153-2164. | |||
| == Applications == | |||
| 3. Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. ''Organic Letters'', '''1999''', ''1'', 953-956. | |||
| Grubbs catalysts are of interest for ].<ref>{{Cite book |title=Olefin metathesis: theory and practice |date=2014 |publisher=Wiley |isbn=978-1-118-71156-9 |editor-last=Grela |editor-first=Karol |location=Hoboken, New Jersey}}</ref><ref>{{Cite journal |last=Matsuo |first=Takashi |date=March 2021 |title=Functionalization of Ruthenium Olefin-Metathesis Catalysts for Interdisciplinary Studies in Chemistry and Biology |journal=Catalysts |language=en |volume=11 |issue=3 |pages=359 |doi=10.3390/catal11030359 |doi-access=free |issn=2073-4344}}</ref> It is mainly applied to fine chemical synthesis. Large-scale commercial applications of olefin metathesis almost always employ heterogeneous catalysts or ill-defined systems based on ruthenium trichloride.<ref name=KO>{{cite encyclopedia|encyclopedia=Kirk-Othmer Encyclopedia of Chemical Technology|author=Lionel Delaude |author2=Alfred F. Noels |year=2005| doi=10.1002/0471238961.metanoel.a01|place=Weinheim|publisher=Wiley-VCH|isbn = 978-0-471-23896-6|chapter = Metathesis}}</ref> | |||
| ==References== | |||
| 4. Trnka, T. M.; Grubbs, R. H. ''Accounts of Chemical Research'', '''2001''', ''34'', 18-29. | |||
| {{Reflist|30em}} | |||
| <!-- # {{Note|Louie}} Louie, J.; Grubbs, R. H. . --> | |||
| 5. Bonser, K. http://science.howstuffworks.com/self-healing-spacecraft1.htm | |||
| {{Ruthenium compounds}} | |||
| 6. Grubbs, R.H. ''Handbook of Metathesis''; Wiley VCH: United Kingdon, 2003. | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 16:22, 22 October 2024
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have also been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.
First-generation Grubbs catalyst

| |

| |
| Names | |
|---|---|
| IUPAC name Benzylidene-bis(tricyclohexylphosphino)-dichlororuthenium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C43H72Cl2P2Ru |
| Molar mass | 822.97 g·mol |
| Appearance | Purple solid |
| Melting point | 153 °C (307 °F; 426 K) (decomposition) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was reported in 1992. It was prepared from RuCl2(PPh3)4 and diphenylcyclopropene.

This initial ruthenium catalyst was followed in 1995 by what is now known as the first-generation Grubbs catalyst. It is synthesized from RuCl2(PPh3)3, phenyldiazomethane, and tricyclohexylphosphine in a one-pot synthesis.

The first-generation Grubbs catalyst was the first well-defined Ru-based catalyst. It is also important as a precursor to all other Grubbs-type catalysts.
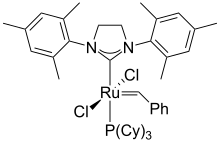
Second-generation Grubbs catalyst
| |

| |
| Names | |
|---|---|
| IUPAC name dichloro(phenylmethylene)(tricyclohexylphosphino)ruthenium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C46H65Cl2N2PRu |
| Molar mass | 848.98 g·mol |
| Appearance | Pinkish brown solid |
| Melting point | 143.5 to 148.5 °C (290.3 to 299.3 °F; 416.6 to 421.6 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H228 |
| Precautionary statements | P210, P240, P241, P280, P378 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
The second-generation catalyst has the same uses in organic synthesis as the first generation catalyst, but generally with higher activity. This catalyst is stable toward moisture and air, thus is easier to handle in laboratories.
Shortly before the discovery of the second-generation Grubbs catalyst, a very similar catalyst based on an unsaturated N-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)imidazole) was reported independently by Nolan and Grubbs in March 1999, and by Fürstner in June of the same year. Shortly thereafter, in August 1999, Grubbs reported the second-generation catalyst, based on a saturated N-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)dihydroimidazole):

In both the saturated and unsaturated cases a phosphine ligand is replaced with an N-heterocyclic carbene (NHC), which is characteristic of all second-generation-type catalysts.
Both the first- and second-generation catalysts are commercially available, along with many derivatives of the second-generation catalyst.
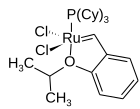
Hoveyda–Grubbs catalysts
| |

| |
| Names | |
|---|---|
| IUPAC name Dichloro(o-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium(II) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H45Cl2OPRu |
| Molar mass | 600.61 g·mol |
| Appearance | Brown solid |
| Melting point | 195 to 197 °C (383 to 387 °F; 468 to 470 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H228 |
| Precautionary statements | P210, P240, P241, P280, P378 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |

| |

| |
| Names | |
|---|---|
| IUPAC name dichloro(o-isopropoxyphenylmethylene)ruthenium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C31H38Cl2N2ORu |
| Molar mass | 626.63 g·mol |
| Appearance | Green solid |
| Melting point | 216 to 220 °C (421 to 428 °F; 489 to 493 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
In the Hoveyda–Grubbs catalysts, the benzylidene ligands have a chelating ortho-isopropoxy group attached to the benzene rings. The ortho-isopropoxybenzylidene moiety is sometimes referred to as a Hoveyda chelate. The chelating oxygen atom replaces a phosphine ligand, which in the case of the 2nd generation catalyst, gives a completely phosphine-free structure. The 1st generation Hoveyda–Grubbs catalyst was reported in 1999 by Amir H. Hoveyda's group, and in the following year, the second-generation Hoveyda–Grubbs catalyst was described in nearly simultaneous publications by the Blechert and Hoveyda laboratories. Siegfried Blechert's name is not commonly included in the eponymous catalyst name. The Hoveyda–Grubbs catalysts, while more expensive and slower to initiate than the Grubbs catalyst from which they are derived, are popular because of their improved stability. By changing the steric and electronic properties of the chelate, the initiation rate of the catalyst can be modulated, such as in the Zhan catalysts. Hoveyda–Grubbs catalysts are easily formed from the corresponding Grubbs catalyst by the addition of the chelating ligand and the use of a phosphine scavenger like copper(I) chloride:
The second-generation Hoveyda–Grubbs catalysts can also be prepared from the 1st generation Hoveyda–Grubbs catalyst by the addition of the NHC:


In one study published by Grubbs and Hong in 2006, a water-soluble Grubbs catalyst was prepared by attaching a polyethylene glycol chain to the imidazolidine group. This catalyst is used in the ring-closing metathesis reaction in water of a diene carrying an ammonium salt group making it water-soluble as well.

Third-generation Grubbs catalyst (fast-initiating catalysts)
The rate of the Grubbs catalyst can be altered by replacing the phosphine ligand with more labile pyridine ligands. By using 3-bromopyridine the initiation rate is increased more than a millionfold. Both pyridine and 3-bromopyridine are commonly used, with the bromo- version 4.8 times more labile resulting in even faster rates. The catalyst is traditionally isolated as a two pyridine complex, however one pyridine is lost upon dissolving and reversibly inhibits the ruthenium center throughout any chemical reaction.
The principal application of the fast-initiating catalysts is as initiators for ring opening metathesis polymerisation (ROMP). Because of their usefulness in ROMP these catalysts are sometimes referred to as the 3rd generation Grubbs catalysts. The high ratio of the rate of initiation to the rate of propagation makes these catalysts useful in living polymerization, yielding polymers with low polydispersity.
Applications
Grubbs catalysts are of interest for olefin metathesis. It is mainly applied to fine chemical synthesis. Large-scale commercial applications of olefin metathesis almost always employ heterogeneous catalysts or ill-defined systems based on ruthenium trichloride.
References
- Grubbs, Robert H. (2003). Handbook of Metathesis (1st ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-30616-9.
- Grubbs, R. H.; Trnka, T. M. (2004). "Ruthenium-Catalyzed Olefin Metathesis". In Murahashi, S. (ed.). Ruthenium in Organic Synthesis. Weinheim: Wiley-VCH. pp. 153–177. doi:10.1002/3527603832.ch6. ISBN 978-3-527-60383-1.
- ^ Vougioukalakis, G. C.; Grubbs, R. H. (2010). "Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts". Chemical Reviews. 110 (3): 1746–1787. doi:10.1021/cr9002424. PMID 20000700.
- Trnka, T. M.; Grubbs, R. H. (2001). "The Development of L2X2Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story". Accounts of Chemical Research. 34 (1): 18–29. doi:10.1021/ar000114f. PMID 11170353.
- Cossy, Janine; Arseniyadis, Stellios; Meyer, Christophe (2010). Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysts (1st ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-32440-8.
- ^ Lionel Delaude; Alfred F. Noels (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 978-0-471-23896-6.
- Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. (1992). "Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media" (PDF). Journal of the American Chemical Society. 114 (10): 3974–3975. doi:10.1021/ja00036a053.
- Schwab, P.; France, M. B.; Ziller, J. W.; Grubbs, R. H. (1995). "A Series of Well-Defined Metathesis Catalysts – Synthesis of and Its Reactions". Angew. Chem. Int. Ed. 34 (18): 2039–2041. doi:10.1002/anie.199520391.
- Schwab, P.; Grubbs, R. H.; Ziller, J. W. (1996). "Synthesis and Applications of RuCl2(=CHR′)(PR3)2: The Influence of the Alkylidene Moiety on Metathesis Activity". J. Am. Chem. Soc. 118 (1): 100–110. doi:10.1021/ja952676d.
- Huang, J.-K.; Stevens, E. D.; Nolan, S. P.; Petersen, J. L. (1999). "Olefin Metathesis-Active Ruthenium Complexes Bearing a Nucleophilic Carbene Ligand". J. Am. Chem. Soc. 121 (12): 2674–2678. doi:10.1021/ja9831352.
- Scholl, M.; Trnka, T. M.; Morgan, J. P.; Grubbs, R. H. (1999). "Increased Ring Closing Metathesis Activity of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with Imidazolin-2-ylidene Ligands". Tetrahedron Letters. 40 (12): 2247–2250. doi:10.1016/S0040-4039(99)00217-8.
- Ackermann, L.; Fürstner, A.; Weskamp, T.; Kohl, F. J.; Herrmann, W. A. (1999). "Ruthenium Carbene Complexes with Imidazolin-2-ylidene Ligands Allow the Formation of Tetrasubstituted Cycloalkenes by RCM". Tetrahedron Lett. 40 (26): 4787–4790. doi:10.1016/S0040-4039(99)00919-3.
- Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. (1999). "Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands". Org. Lett. 1 (6): 953–956. doi:10.1021/ol990909q. PMID 10823227.
- Kingsbury, Jason S.; Harrity, Joseph P. A.; Bonitatebus, Peter J.; Hoveyda, Amir H. (1999). "A Recyclable Ru-Based Metathesis Catalyst". Journal of the American Chemical Society. 121 (4): 791–799. doi:10.1021/ja983222u.
- ^ Gessler, S.; Randl, S.; Blechert, S. (2000). "Synthesis and metathesis reactions of phosphine-free dihydroimidazole carbene ruthenium complex". Tetrahedron Letters. 41 (51): 9973–9976. doi:10.1016/S0040-4039(00)01808-6.
- ^ Garber, S. B.; Kingsbury, J. S.; Gray, B. L.; Hoveyda, A. H. (2000). "Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts". Journal of the American Chemical Society. 122 (34): 8168–8179. doi:10.1021/ja001179g.
- Antonova, A. S.; Zubkov, F. I. (2024). "Hoveyda-Grubbs type complexes with ruthenium-pnictogen/halcogen/halogen coordination bond. Synthesis, catalytic activity, applications". Russian Chemical Reviews. 93 (8): RCR5132. doi:10.59761/rcr5132. Retrieved 2024-10-22.
- Engle, Keary M.; Lu, Gang; Luo, Shao-Xiong; Henling, Lawrence M.; Takase, Michael K.; Liu, Peng; Houk, K. N.; Grubbs, Robert H. (2015). "Origins of Initiation Rate Differences in Ruthenium Olefin Metathesis Catalysts Containing Chelating Benzylidenes". Journal of the American Chemical Society. 137 (17): 5782–5792. doi:10.1021/jacs.5b01144. PMID 25897653.
- Luo, Shao-Xiong; Engle, Keary M.; Deng, Xiaofei; Hejl, Andrew; Takase, Michael K.; Henling, Lawrence M.; Liu, Peng; Houk, K. N.; Grubbs, Robert H. (2018). "An Initiation Kinetics Prediction Model Enables Rational Design of Ruthenium Olefin Metathesis Catalysts Bearing Modified Chelating Benzylidenes". ACS Catalysis. 8 (5): 4600–4611. doi:10.1021/acscatal.8b00843. PMC 7289044. PMID 32528741.
- Grubbs, Robert H.; Hong, Soon Hyeok (2006). "Highly Active Water-Soluble Olefin Metathesis Catalyst" (PDF). Journal of the American Chemical Society. 128 (11): 3508–3509. doi:10.1021/ja058451c. PMID 16536510.
- Love, J. A.; Morgan, J. P.; Trnka, T. M.; Grubbs, R. H. (2002). "A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile". Angew. Chem. Int. Ed. Engl. 41 (21): 4035–4037. doi:10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. PMID 12412073.
- Walsh, Dylan J.; Lau, Sii Hong; Hyatt, Michael G.; Guironnet, Damien (2017-09-25). "Kinetic Study of Living Ring-Opening Metathesis Polymerization with Third-Generation Grubbs Catalysts". Journal of the American Chemical Society. 139 (39): 13644–13647. doi:10.1021/jacs.7b08010. PMID 28944665.
- Leitgeb, Anita; Wappel, Julia; Slugovc, Christian (2010). "The ROMP toolbox upgraded". Polymer. 51 (14): 2927–2946. doi:10.1016/j.polymer.2010.05.002.
- Choi, T.-L.; Grubbs, R. H. (2003). "Controlled Living Ring-Opening-Metathesis Polymerization by a Fast-Initiating Ruthenium Catalyst". Angewandte Chemie International Edition. 42 (15): 1743–1746. doi:10.1002/anie.200250632. PMID 12707895.
- Grela, Karol, ed. (2014). Olefin metathesis: theory and practice. Hoboken, New Jersey: Wiley. ISBN 978-1-118-71156-9.
- Matsuo, Takashi (March 2021). "Functionalization of Ruthenium Olefin-Metathesis Catalysts for Interdisciplinary Studies in Chemistry and Biology". Catalysts. 11 (3): 359. doi:10.3390/catal11030359. ISSN 2073-4344.
| Ruthenium compounds | |
|---|---|
| Ru(0) | |
| Ru(I) | |
| Ru(II) | |
| Ru(II,III) | |
| Ru(III) |
|
| Ru(IV) | |
| Ru(V) | |
| Ru(VI) | |
| Ru(VII) | |
| Ru(VIII) | |