| Revision as of 11:14, 9 April 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (← Previous edit | Latest revision as of 15:34, 19 April 2024 edit undoReconrabbit (talk | contribs)Autopatrolled, Extended confirmed users, New page reviewers8,375 editsm →Use: typoTag: Visual edit | ||
| (52 intermediate revisions by 33 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Distinguish|Bisulfite}} | {{Distinguish|Bisulfite}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 423158544 | ||
| | Name = Disulfite ion | | Name = Disulfite ion | ||
| | ImageFile = |
| ImageFile = Structure of metabisulfite ion.png | ||
| | ImageSize = |
| ImageSize = 190px | ||
| | ImageFile1 = | | ImageFile1 = Metabisulfite-ion-from-xtal-3D-bs-17.png | ||
| ⚫ | | IUPACName = disulfite<ref name=redbookname>{{RedBookRef|page=130}}</ref> | ||
| | ImageSize1 = | |||
| | SystematicName = pentaoxido-1''κ''<sup>3</sup>''O'',2''κ''<sup>2</sup>''O''-disulfate(S—S)(2−)<ref name=redbookname/> | |||
| | ImageName1 = | |||
| ⚫ | | IUPACName = disulfite |
||
| | OtherNames = metabisulfite ion<br />pyrosulfite | | OtherNames = metabisulfite ion<br />pyrosulfite | ||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | CASNo = | | CASNo = 23134-05-6 | ||
| | CASNo_Ref = | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | ChemSpiderID = 140610 | |||
| | PubChem = 159940 | | PubChem = 159940 | ||
| | RTECS = | | RTECS = | ||
| | UNII = 7992SO049K | |||
| | SMILES = ()()() | |||
| | StdInChI=1S/H2O5S2/c1-6(2)7(3,4)5/h(H,1,2)(H,3,4,5)/p-2 | |||
| | StdInChIKey = WBZKQQHYRPRKNJ-UHFFFAOYSA-L | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = S |
| Formula = {{chem|S|2|O|5|2−}} | ||
| | MolarMass = | | MolarMass = | ||
| | Appearance = | | Appearance = | ||
| Line 25: | Line 29: | ||
| | MeltingPt = | | MeltingPt = | ||
| | BoilingPt = | | BoilingPt = | ||
| | ConjugateAcid = ] | |||
| }} | }} | ||
| }} | }} | ||
| A '''disulfite''', commonly known as '''metabisulfite''' or '''pyrosulfite''', is a ] containing the ion {{chem|S|2|O|5|2−}}. It is a colorless dianion that is primarily marketed in the form of ] or ]. When dissolved in water, these salts release the hydrogensulfite {{chem|H|S|O|3|−}} anion. These salts act equivalently to ] or ].<ref>{{cite book|doi=10.1002/9780470132333.ch49|isbn=9780470132333|chapter=Sulfites and Pyrosulfites of the Alkali Metals|series=Inorganic Syntheses|year=1946|last1=Johnstone|first1=H. F.|title=Inorganic Syntheses|pages=162–167|volume=2}}</ref> | |||
| A '''disulfite''', commonly known as '''metabisulfite''', is a ] containing the '''disulfite ion''' ('''metabisulfite ion''') . | |||
| == |
==Structure== | ||
| In contrast to ] ({{chem|S|2|O|7|2−}}), disulfite ion ({{chem|S|2|O|5|2−}}) has an unsymmetrical structure with an S-S bond. The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3.<ref>{{cite journal|first1=I.|last1=Lindqvist|first2=M.|last2=Mörtsell|title=The Structure of Potassium Pyrosulfite and the Nature of the Pyrosulfite Ion". |journal=] |date= 1957|volume=10 |pages=406–409 |doi=10.1107/S0365110X57001322 |doi-access=free}}</ref> | |||
| ⚫ | ===Production |
||
| The anion consists of an SO<sub>2</sub> group linked to an SO<sub>3</sub> group, with the negative charge more localized on the SO<sub>3</sub> end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.<ref>K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" ''Acta Crystallogr.'' (2004). '''B60''', 155–162. {{doi|10.1107/S0108768104003325}}</ref> | |||
| The disulfite ion is a ] of the ] ion (HSO<sub>3</sub><sup>−</sup>). It can arise from: | |||
| ⚫ | ===Production=== | ||
| Salts of disulfite ion are produced by dehydration of salts of ] ion ({{chem|HSO|3|−}}). When solutions of ] or ] are evaporated, ] and ] result.<ref>{{Ullmann|doi=10.1002/14356007.a25_477|isbn=3527306730|title=Sulfites, Thiosulfates, and Dithionitesl Chemistry|year=2000|last1=Barberá|first1=José Jiménez|last2=Metzger|first2=Adolf|last3=Wolf|first3=Manfred}}</ref> | |||
| :{{chem2|2 HSO3−}} ] {{chem2|S2O5(2−) + H2O}} | |||
| ⚫ | Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.<ref name=Bassam>Bassam Z. Shakhashiri: The University of Wisconsin Press, 1992, p.9</ref> | ||
| ''']''' | |||
| ⚫ | Disulfite is the conjugate base of ] (pyrosulfurous acid), which originates from ] in accordance with the dehydration reaction above:<br /> | ||
| In aqueous solution, the disulfite ion is formed in minor amounts by dehydration of bisulfite in an equilibrium: | |||
| : |
:2 H<sub>2</sub>SO<sub>3</sub> → 2 {{chem|HSO|3|−}} + 2 H<sup>+</sup> → H<sub>2</sub>S<sub>2</sub>O<sub>5</sub> + H<sub>2</sub>O | ||
| ⚫ | The disulfite ion also arises from the addition of ] to the ] ion: | ||
| ⚫ | Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.<ref name=Bassam>Bassam Z. Shakhashiri: The University of Wisconsin Press |
||
| ⚫ | |||
| :2 H<sub>2</sub>SO<sub>3</sub> → 2 HSO<sub>3</sub><sup>−</sup> + 2 H<sup>+</sup> → H<sub>2</sub>S<sub>2</sub>O<sub>5</sub> + H<sub>2</sub>O | |||
| '''addition''' | |||
| ⚫ | The disulfite ion also arises from the addition of ] to the ] ion: |
||
| {| class="wikitable" | {| class="wikitable" | ||
| |- | |- | ||
| |HSO |
|{{chem|HSO|3|−}} ] {{chem|SO|3|2−}} + H<sup>+</sup><br /><br />SO<sub>3</sub><sup>2−</sup> + SO<sub>2</sub> ] {{chem|S|2|O|5|2−}} || || ] | ||
| |} | |} | ||
| ==Use== | |||
| ===Other reactions=== | |||
| Disulfite salts are used for ] and as ], with the main species used for this purpose being ] (])<ref>{{Cite journal |last=Noorafshan |first=A. |last2=Asadi-Golshan |first2=R. |last3=Monjezi |first3=S. |last4=Karbalay-Doust |first4=S. |date=2014 |title=Sodium metabisulphite, a preservative agent, decreases the heart capillary volume and length, and curcumin, the main component of Curcuma longa, cannot protect it |url=https://pubmed.ncbi.nlm.nih.gov/25629268 |journal=Folia Biologica |volume=60 |issue=6 |pages=275–280 |issn=0015-5500 |pmid=25629268}}</ref> and ] (E224).<ref>{{Cite web |last=PubChem |title=Potassium metabisulfite |url=https://pubchem.ncbi.nlm.nih.gov/compound/28019 |access-date=2024-04-19 |website=pubchem.ncbi.nlm.nih.gov |language=en}}</ref> Sulfites are implicated in asthmatic reactions and may also cause symptoms in non-asthmatic individuals, namely ], ], ], ], ] and ], and even life-threatening ].<ref name="pmid24834193">{{cite journal |vauthors=Vally H, Misso NL |title=Adverse reactions to the sulphite additives |journal=Gastroenterol Hepatol Bed Bench |volume=5 |issue=1 |pages=16–23 |date=2012 |pmid=24834193 |pmc=4017440 |doi= |url=}}</ref> | |||
| In aqueous solution, disulfite salts decompose with acids:<br /> | |||
| S<sub>2</sub>O<sub>5</sub><sup>2−</sup> + H<sup>+</sup> → HSO<sub>3</sub><sup>−</sup> + SO<sub>2</sub> | |||
| ==Examples of disulfites== | |||
| *] (]) and ] (E224) are used as a ] and ] in food. | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 15:34, 19 April 2024
Not to be confused with Bisulfite.
| |

| |
| Names | |
|---|---|
| IUPAC name disulfite | |
| Systematic IUPAC name pentaoxido-1κO,2κO-disulfate(S—S)(2−) | |
| Other names
metabisulfite ion pyrosulfite | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | S 2O 5 |
| Conjugate acid | Disulfurous acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.
Structure
In contrast to disulfate (S
2O
7), disulfite ion (S
2O
5) has an unsymmetrical structure with an S-S bond. The oxidation state of the sulfur atom bonded to 3 oxygen atoms is +5 while oxidation number of other sulfur atom is +3.
The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.
Production
Salts of disulfite ion are produced by dehydration of salts of hydrogensulfite ion (HSO
3). When solutions of sodium hydrogensulfite or potassium hydrogensulfite are evaporated, sodium metabisulfite and potassium metabisulfite result.
Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.
Disulfite is the conjugate base of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above:
- 2 H2SO3 → 2 HSO
3 + 2 H → H2S2O5 + H2O
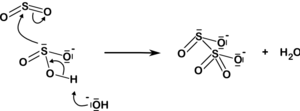
The disulfite ion also arises from the addition of sulfur dioxide to the sulfite ion:
| HSO 3 3 + H SO3 + SO2 2O 5 |
 |
Use
Disulfite salts are used for preserving food and beverages and as antioxidants, with the main species used for this purpose being sodium metabisulfite (E223) and potassium metabisulfite (E224). Sulfites are implicated in asthmatic reactions and may also cause symptoms in non-asthmatic individuals, namely dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, and even life-threatening anaphylaxis.
References
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. Inorganic Syntheses. Vol. 2. pp. 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- Lindqvist, I.; Mörtsell, M. (1957). "The Structure of Potassium Pyrosulfite and the Nature of the Pyrosulfite Ion"". Acta Crystallographica. 10: 406–409. doi:10.1107/S0365110X57001322.
- K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" Acta Crystallogr. (2004). B60, 155–162. doi:10.1107/S0108768104003325
- Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477. ISBN 3527306730.
- Bassam Z. Shakhashiri: Chemical demonstrations: a handbook for teachers of chemistry The University of Wisconsin Press, 1992, p.9
- Noorafshan, A.; Asadi-Golshan, R.; Monjezi, S.; Karbalay-Doust, S. (2014). "Sodium metabisulphite, a preservative agent, decreases the heart capillary volume and length, and curcumin, the main component of Curcuma longa, cannot protect it". Folia Biologica. 60 (6): 275–280. ISSN 0015-5500. PMID 25629268.
- PubChem. "Potassium metabisulfite". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-04-19.
- Vally H, Misso NL (2012). "Adverse reactions to the sulphite additives". Gastroenterol Hepatol Bed Bench. 5 (1): 16–23. PMC 4017440. PMID 24834193.